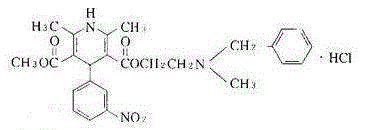
Nicardipine hydrochloride sodium chloride injection and preparation method thereof
A technology of nicardipine hydrochloride and dipine sodium chloride, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., can solve the problems of easy generation of decomposition products, poor thermal stability, etc., and achieve stable properties and easy Storage, Quality and Reliable Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of Nicardipine Hydrochloride Sodium Chloride Injection
[0033] Weigh 10.76g nicardipine hydrochloride, 900g sodium chloride, 1000g mannitol, dissolve in 90000ml water for injection.
[0034] Add glacial acetic acid to adjust the pH to 4.0.
[0035] Add water for injection to a total volume of 100000ml.
[0036] Add needles to decolorize with activated charcoal for 30 min.
[0037] Filter and decarbonize, take samples to measure the pH value and content, and the pH and the content of each component are in line with the standard;
[0038] Fine filter with 0.2μm microporous membrane;
[0039] Fill to 100ml glass infusion bottle, 100ml per bottle, crimp the cap;
[0040] Sterilize at 121°C for 8 minutes, and cool down to room temperature within 30 minutes after sterilization.
Embodiment 2
[0041] Example 2 Preparation of Nicardipine Hydrochloride Sodium Chloride Injection
[0042] Weigh 21.52g nicardipine hydrochloride, 900g sodium chloride, 1000g mannitol, dissolve in 90000ml water for injection.
[0043] Add glacial acetic acid to adjust the pH to 4.0.
[0044] Add water for injection to a total volume of 100000ml.
[0045] Add needles to decolorize with activated charcoal for 30 min.
[0046] Filter and decarbonize, take samples to measure the pH value and content, and the pH and the content of each component are in line with the standard;
[0047] Fine filter with 0.2μm microporous membrane;
[0048] Fill to 100ml glass infusion bottle, 100ml per bottle, crimp the cap;
[0049] Sterilize at 121°C for 8 minutes, and cool down to room temperature within 30 minutes after sterilization.
Embodiment 3
[0050] Example 3 pH Stability
[0051] Nicardipine hydrochloride injection is sensitive to light and heat, and the color of the injection will become darker if it is exposed to light or heated for a long time. And nicardipine hydrochloride injection increases with pH value, and stability reduces. The pH stability of Nicardipine Hydrochloride Sodium Chloride Injection of the present invention is tested, method: prepare the Nicardipine Hydrochloride Sodium Chloride Injection of different pH values respectively according to the prescription of Example 2, place in 80 ℃ oven 10 days, the results are shown in Table 1.
[0052] Table 1 pH stability of nicardipine hydrochloride sodium chloride injection
[0053] pH value Decomposition products + related substances (%) 3.0 >3.0 3.5 <3.0
[0054] It can be seen from the results in Table 1 that nicardipine hydrochloride is the most stable around pH 4.0, and the stability is reduced if the pH is too high or too lo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com