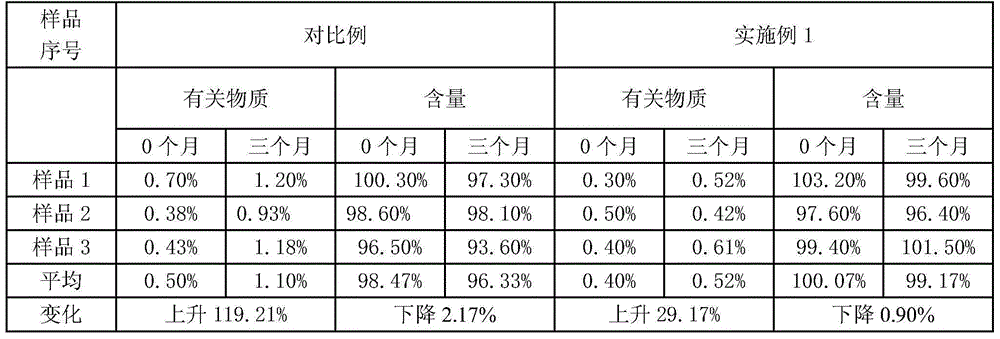
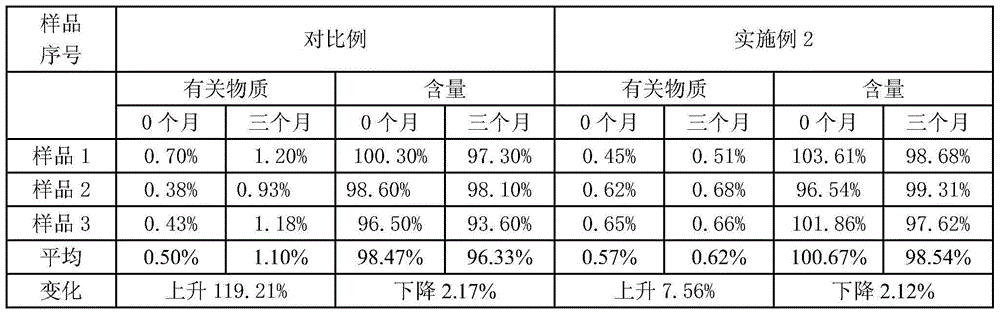
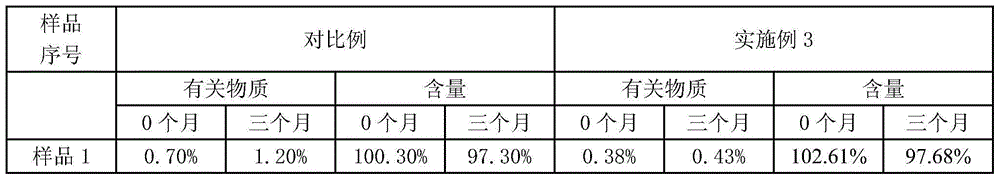
Pantoprazole sodium enteric micro-pellet capsules and preparation method thereof
A technology of pantoprazole sodium enteric and soluble pellets, which can be used in capsule delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve problems such as poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The preparation method of described pantoprazole sodium enteric-coated pellet capsule, comprises the following steps:
[0057] Each component is weighed according to the weight ratio of the components of each layer;
[0058] 1) Preparation of the main drug layer:
[0059] Under the condition of stirring, add hydroxypropyl methylcellulose into purified water, after completely dissolving, add sodium carbonate, pantoprazole sodium, sodium lauryl sulfate, Tween-80 in sequence, and get the main drug layer after stirring evenly Coating solution;
[0060] Place the blank pellet core in a pellet coating machine, and coat it with the main drug layer coating solution to obtain pellets containing the main drug layer;
[0061] 2) Wrap the first isolation layer:
[0062] Under the condition of stirring, add hydroxypropyl methylcellulose into purified water, after completely dissolving, add talcum powder, sodium carbonate, titanium dioxide in turn, after stirring evenly, grind in ...
Embodiment 1
[0078] A kind of preparation of pantoprazole sodium enteric-coated pellet capsule:
[0079] By weight, weigh the following components:
[0080] Main drug layer:
[0081] blank core
35.89 copies
5.82 copies
1.75 servings
0.40 servings
0.35 parts
Tween 80
0.70 servings
50 copies
[0082] First isolation layer:
[0083] Hypromellose
3.90 servings
0.20 servings
0.03 parts
Titanium dioxide
0.70 servings
purified water
40 copies
[0084] Second isolation layer:
[0085] Hypromellose
3.90 servings
0.20 servings
Titanium dioxide
0.70 servings
[0086] purified water
40 copies
[0087] Enteric layer:
[0088] triethyl citrate ...
Embodiment 2
[0109] A kind of preparation of pantoprazole sodium enteric-coated pellet capsule:
[0110] By weight, weigh the following components:
[0111] Main drug layer:
[0112] blank core
35 copies
Pantoprazole Sodium
5.5 servings
Hypromellose
2 copies
0.3 parts
0.4 parts
Tween 80
0.6 parts
purified water
40 copies
[0113] First isolation layer:
[0114] Hypromellose
3.5 servings
Talc powder
0.25 parts
0.025 copies
Titanium dioxide
0.75 parts
purified water
30 copies
[0115] Second isolation layer:
[0116] Hypromellose
4.5 servings
Talc powder
0.25 parts
[0117] Titanium dioxide
0.75 parts
purified water
30 copies
[0118] Enteric layer:
[0119] triethyl citrate
1 copy
Talc powder...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com