Completely inactivated vaccine for preventing and treating spinach frog skin rot and preparation method thereof
A technology for inactivated vaccines and rotten skin diseases, applied in skin diseases, bacterial antigen components, antibacterial drugs, etc., can solve problems such as lack of uniformity, and achieve the effects of low production cost, good promotion and use value, and convenient use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, screening, identification of pathogenic bacteria, carry out the following steps successively:
[0038] 1) Rinse the body surface of the diseased and dying frog with sterile water, disinfect with 75% (volume%) alcohol, and take the deep skin of the head, legs, and the rotten skin around the excretory hole and the liver tissue of the diseased frog. , respectively, were coated in LB solid medium and incubated at 30°C for 24h. Colonies of different morphologies were picked for further streaking until pure cultures were obtained.
[0039] LB solid medium is a conventional medium, as follows: (both mass concentration g / L): peptone 20g, K 2 HPO 4 .3H 2 O2.5g, MgSO 4 0.73g, glycerol 15.0ml, agar 15.0g, distilled water to 1.0L; pH 7.0, 121℃, 15min sterilization.
[0040] 2), using TaKaRa 16S rDNA Bacterial Identification PCR Kit (purchased from Dalian Bao Bioengineering Co., Ltd.) to extract the genomic DNA of each strain from the single colony obtained in the...
Embodiment 2
[0044] Example 2. Regression infection experiment
[0045] Follow these steps in order:
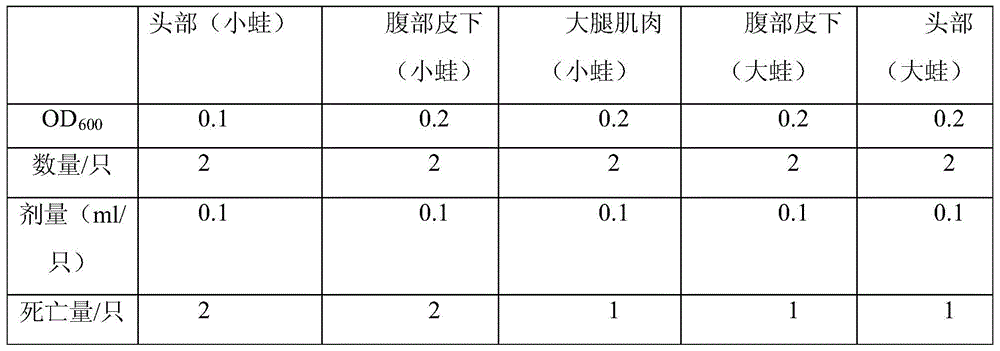
[0046] 1), inoculate the above-obtained main pathogenic bacteria (that is, Acinetobacter genus) in LB liquid medium, after 30 ℃ of expanded culture 16h, prepare OD with sterile physiological saline 600 The mixed infection solution with values of 0.1 and 0.2. There were 10 healthy frogs in the experimental group and the control group. After alcohol disinfection, the experimental group was injected with the bacterial solution, and the control group was injected with the same amount of normal saline (Table 1). Cultivated in a 25°C culture tank, observed and recorded the incidence, dissected the dying frogs, and isolated and identified pathogenic bacteria (Table 2).
[0047] Table 1 Regression infection experiment
[0048]
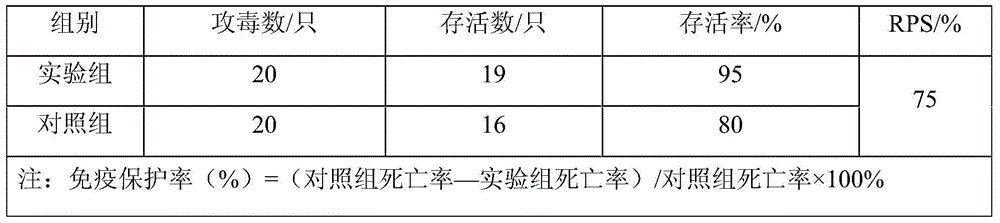
[0049] The results of regression infection showed that Acinetobacter strains had strong pathogenicity to P. spinosa, and the mortality rate of P. spinosa in the e...
Embodiment 3
[0054] Example 3. Specific immune verification
[0055] 1), with Acinetobacter jelly, Acinetobacter lwoutii, and Acinetobacter johnsonii as the main pathogenic bacteria, carry out expanded culture.
[0056] Prepared to OD with sterile water 600 270mL of mixed infection solution with a value of 0.2. Remarks: Acinetobacter agarinii, Acinetobacter lwoutii, and Acinetobacter johnsonii have the same concentration in the mixed infection solution.
[0057] 2), get 8 healthy frogs, soak in the infection bacterial liquid, soak for 3 days in total, and set the control group of equal amount to soak in sterile water in the same way.
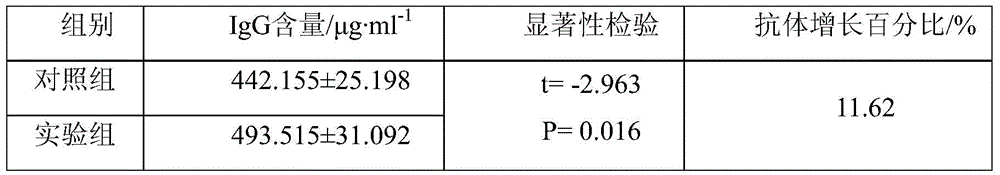
[0058] 3) After soaking, random sampling was performed to collect serum, and an immunoglobulin IgG content assay kit was used to determine the antibody IgG content in the experimental group and the control group.
[0059] Results: The antibody content in the experimental group was generally higher than that in the healthy frogs in the control group. There...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com