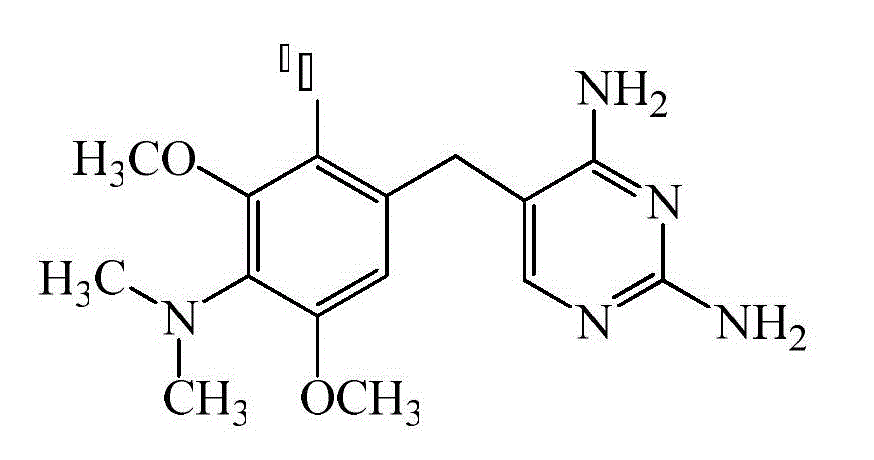
Method for preparing tritium-labeling aditoprim
A labeling and acid anhydride technology, which is applied in the field of preparation of tritium-labeled ediprine, can solve the problems of high cost of radioactive waste, long half-life, environmental pollution and the like, and achieves a simple and easy synthesis route, reduced environmental pollution and high chemical purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
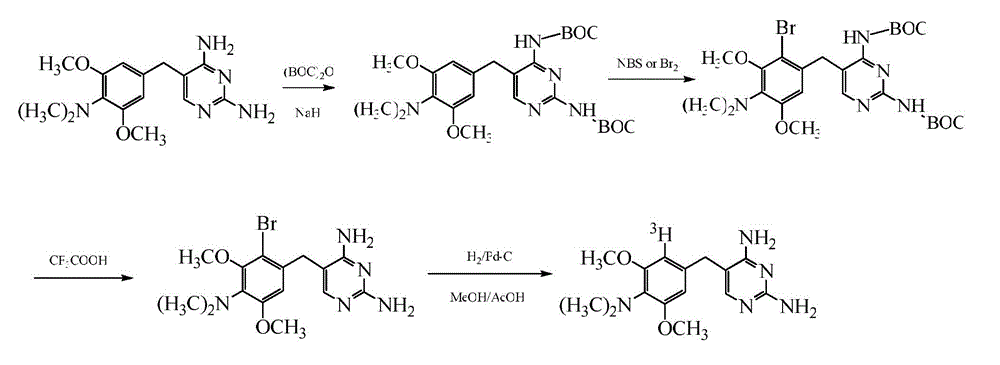
[0030](1) Add 60mL of dichloromethane, 8.0g (26.5mmol) of elliprine and 1.0g (41mmol) of sodium hydride that have been treated in advance into a 100mL round bottom equipped with a magnet, stir to dissolve and add dichloromethane Tert-butyl dicarbonate (BOC anhydride) 11.5g (53.0mmol), stirred at 25°C for 12 hours, poured the reaction solution into 50mL ice water, extracted in a separatory funnel, took the dichloromethane layer, added anhydrous sulfuric acid After drying with sodium, it was filtered, concentrated under reduced pressure and recrystallized with a small amount of ethyl acetate to obtain 8.5 g of BOC-protected ideproline.
[0031] (2) Dissolve 4g (8mmol) of BOC anhydride-protected ideproline obtained in step (1) in 50mL of dichloromethane, and dissolve 2.53g (16mmol) of liquid bromine in 10mL of dichloromethane. (0~5°C), it was slowly added dropwise into dichloromethane of elliprine, and after the dropwise addition was completed, it was stirred and reacted for 4 ho...
Embodiment 2
[0036] (1) Add 60mL of tetrahydrofuran, 8.0g (26.5mmol) of ideproline and 1.0g (41mmol) of sodium hydride that have been treated in advance into a 100mL round bottom equipped with a magnet, stir to dissolve and add di-tert-butyl Base dicarbonate (BOC anhydride) 23.0g (105.5mmol), stirred and reacted at 25°C for 12 hours, poured the reaction solution into 200mL ice water, extracted with dichloromethane in a separatory funnel, took the dichloromethane layer, added After drying with sodium sulfate, it was filtered, concentrated under reduced pressure, and then recrystallized with a small amount of ethyl acetate to obtain 8.1 g of BOC-protected ideproline.
[0037] (2) Dissolve 4g (8mmol) of BOC anhydride-protected ideproline obtained in step (1) in 50mL of dichloromethane, and dissolve 2.53g (16mmol) of liquid bromine in 10mL of dichloromethane. (0~5°C), it was slowly added dropwise into dichloromethane of elliprine, and after the dropwise addition was completed, it was stirred a...
Embodiment 3
[0042] (1) Add 60mL of tetrahydrofuran, 8.0g (26.5mmol) of ideproline and 2.12g (53mmol) of sodium hydroxide to a 100mL round bottom equipped with a magnet, stir and dissolve, then add di-tert-butyl dicarbonate (BOC anhydride) 23.0g (105.5mmol), stirred and reacted at 25°C for 12 hours, poured the reaction liquid into 200mL ice water, extracted with dichloromethane in a separating funnel, took the dichloromethane layer, added anhydrous sodium sulfate to dry Afterwards, it was filtered, concentrated under reduced pressure, and then recrystallized with a small amount of ethyl acetate to obtain 7.8 g of BOC-protected ideproline.
[0043] (2) Dissolve 4g (8mmol) of BOC anhydride-protected ideproline obtained in step (1) in 50mL of dichloromethane, and dissolve 2.53g (16mmol) of liquid bromine in 10mL of dichloromethane. (0~5°C), it was slowly added dropwise into dichloromethane of elliprine, and after the dropwise addition was completed, it was stirred and reacted for 4 hours at 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com