Preparation method of medicinal crystal form tofacitinib citrate
A technology of tofacitinib and citric acid, which is applied in the field of preparation of pharmaceutical crystalline tofacitinib citrate, can solve the problem of increased changes in impurities, insoluble finished products, and unrepeatable temperature and solvent volume. Example and other issues to achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Put 70g of tofacitinib citrate raw material into the reactor, and at the same time vacuum pump 0.7kg of ethanol aqueous solution, wherein the mass ratio of isopropanol and water is 1:1; slowly heat to about 60°C to dissolve, then keep stirring After 2 hours, a clear and transparent solution was obtained; then it took 2 hours to slowly cool down to 15°C, and heat and stir for 4 hours to crystallize; the precipitated crystals were centrifuged and dried to obtain 63g off-white wet product, which was dried under reduced pressure and vacuum at 50°C for 8 Hours, 60 g of dry white solid were obtained.
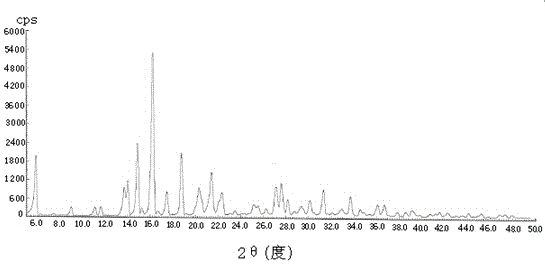
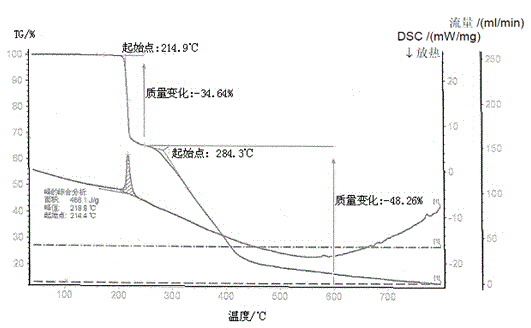
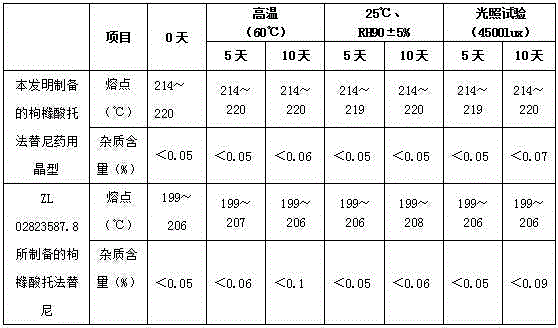
[0032] It is detected by X-ray diffraction, and its X-ray pattern is as follows figure 1 As shown, it shows that the white solid is pharmaceutical crystalline form of tofacitinib citrate; the melting point of the crystal was determined by differential scanning calorimetry (TGA-DSC), and its melting point was 214-220 °C, and its TGA-DSC spectrum Such as figure 2 shown. In ad...
Embodiment 2
[0034] Drop into 70g tofacitinib citrate crude drug in reactor, simultaneously vacuum pump into 1.05kg isopropanol aqueous solution, wherein the mass ratio of isopropanol and water is 2:1, slowly heat to about 80 ℃ and make it dissolve, then Insulated and stirred for 1 hour to obtain a clear and transparent solution; then it took 2 hours to slowly cool down to about 15°C, heat and stir for 4 hours to crystallize; centrifuge and dry it to obtain 65g off-white wet product, and vacuum dry it at 30°C for 8 hours , to obtain 61g of dry white medicinal crystal form tofacitinib citrate solid.
Embodiment 3
[0036] In the reactor, drop into 70g tofacitinib citrate crude drug, simultaneously vacuum pump into 1.4kg isopropanol aqueous solution, wherein the mass ratio of isopropanol and water is 3:1, slowly heat to about 95 ℃ and make it dissolve, then Insulated and stirred for 2 hours, a clear and transparent solution was obtained; it took 1 hour to slowly cool down to 10°C, heat-preserved and stirred for 8 hours to crystallize; it was centrifuged and dried to obtain 66g of off-white wet product, and dried under reduced pressure and vacuum at 60°C for 8 hours , to obtain 59g of dry white medicinal crystal form tofacitinib citrate solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com