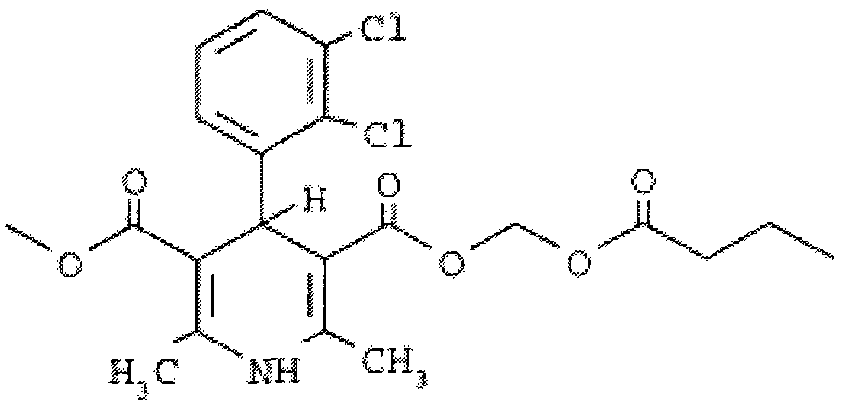
Clevidipine butyrate composition for injection and preparation method thereof
A technology of clevidipine butyrate and composition, which is applied in the field of clevidipine butyrate emulsion for injection and its preparation, can solve problems such as easy collapse, decreased drug encapsulation rate, increased drug leakage, etc. Effect of improving encapsulation rate and increasing operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
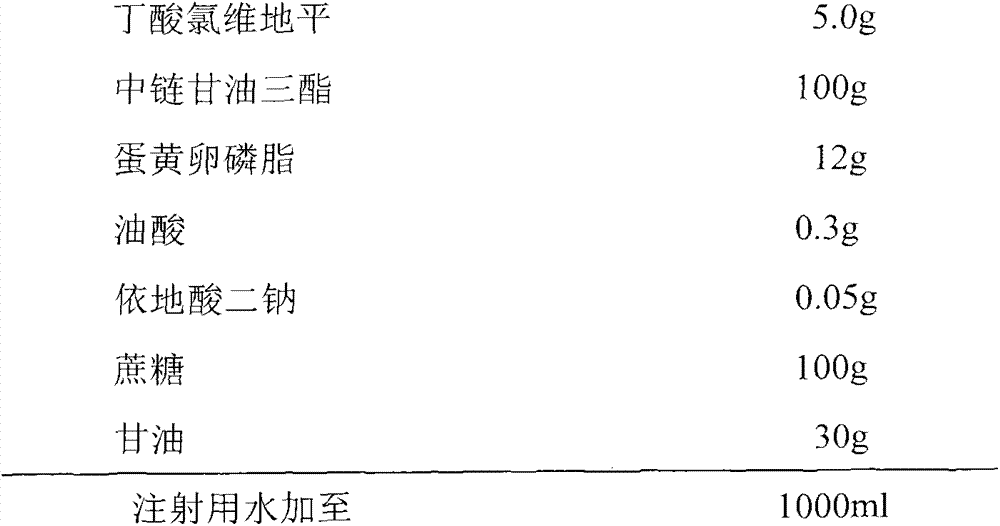
[0039] prescription:
[0040]
[0041] crafting process:
[0042] (1) Preparation of the water phase: add sucrose and edetate disodium into water to dissolve, heat to 65°C, and set aside;
[0043] (2) Preparation of the oil phase: heating medium-chain triglycerides to 65°C, adding egg yolk lecithin, oleic acid to dissolve, adding clevidipine butyrate, and stirring to dissolve;
[0044] (3) Preparation of colostrum: Add the oil phase of step (2) into the water phase of step (1), at a temperature of 65°C, high-speed shear dispersion, shear speed of 10,000 rpm, and time of 15 minutes, to form colostrum
[0045] (4) High-pressure homogenization: Step (3) is subjected to high-pressure homogenization by a micro-jet homogenizer twice, with a pressure of 1000 to 1200 bar;
[0046] (5) Preliminary filtration: Prefilter the refined milk in step (4) through a 0.45 μm filter membrane;
[0047] (6) Sterile filtration: the emulsion obtained in step (5) is sterilized by filtration thro...
Embodiment 2
[0051] Comparative example 1 and the comparison of embodiment 1
[0052] (1) Appearance evaluation
[0053] It is better to maintain the original volume, not collapse, not shrink, the surface is smooth, and it can fall off in one piece but not broken. For color evaluation, it is better to have uniform color, no mottle, and fine texture.
[0054] (2) Evaluation of redispersibility
[0055] Take the freeze-dried products of each prescription, add 1mL of water for injection, and shake to disperse. It is better to disperse quickly to obtain a uniform solution after shaking. The less the number of shakes, the better the redispersibility.
[0056] (3) Determination of Encapsulation Efficiency
[0057] Determination of encapsulation efficiency by ultrafiltration centrifugation
[0058] ①Choose a 10K ultrafiltration centrifuge tube and centrifuge at 3000rpm for 5 minutes to measure the particle size distribution of the viscous upper layer and whether there are particles in the s...
Embodiment 3
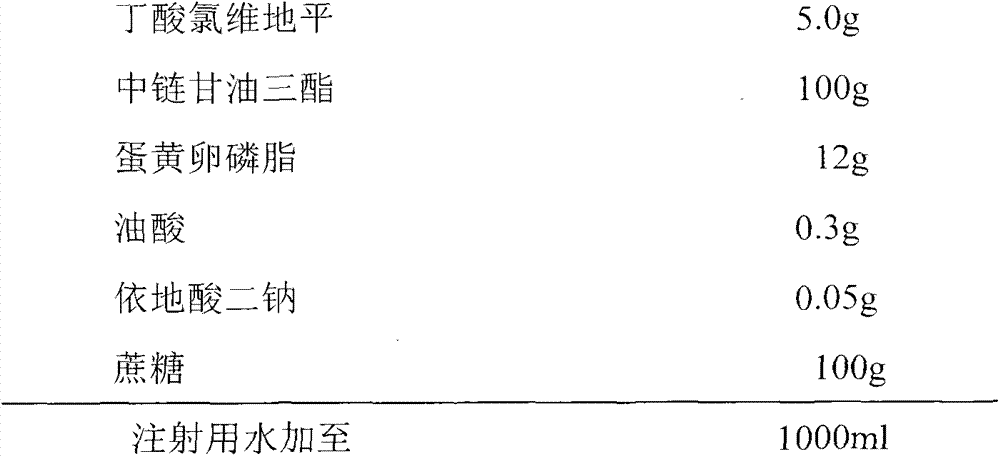
[0072] prescription:
[0073]
[0074] crafting process:
[0075] (1) Preparation of the water phase: add lactose and edetate disodium into water to dissolve, heat to 55°C, and set aside;
[0076] (2) Preparation of the oil phase: heating medium-chain triglycerides to 55°C, adding egg yolk lecithin, oleic acid to dissolve, adding clevidipine butyrate, and stirring to dissolve;
[0077] (3) Preparation of colostrum: Add the oil phase of step (2) into the water phase of step (1), at a temperature of 55°C, high-speed shear dispersion, shear speed of 8000rpm, and time of 30 minutes to form colostrum
[0078] (4) High-pressure homogenization: Step (3) is homogenized by a micro-jet homogenizer for 3 times at a pressure of 800 to 1000 bar;
[0079] (5) Preliminary filtration: Prefilter the refined milk in step (4) through a 0.45 μm filter membrane;
[0080] (6) Sterile filtration: the emulsion obtained in step (5) is sterilized by filtration through a 0.22 μm microporous membra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com