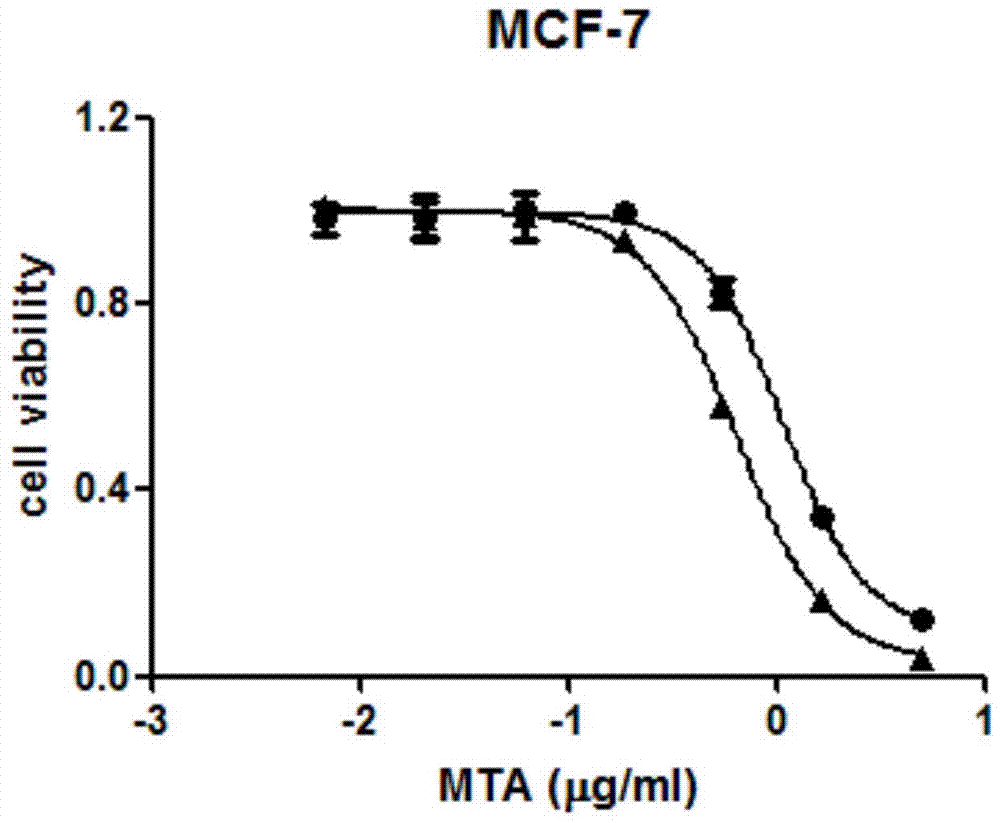
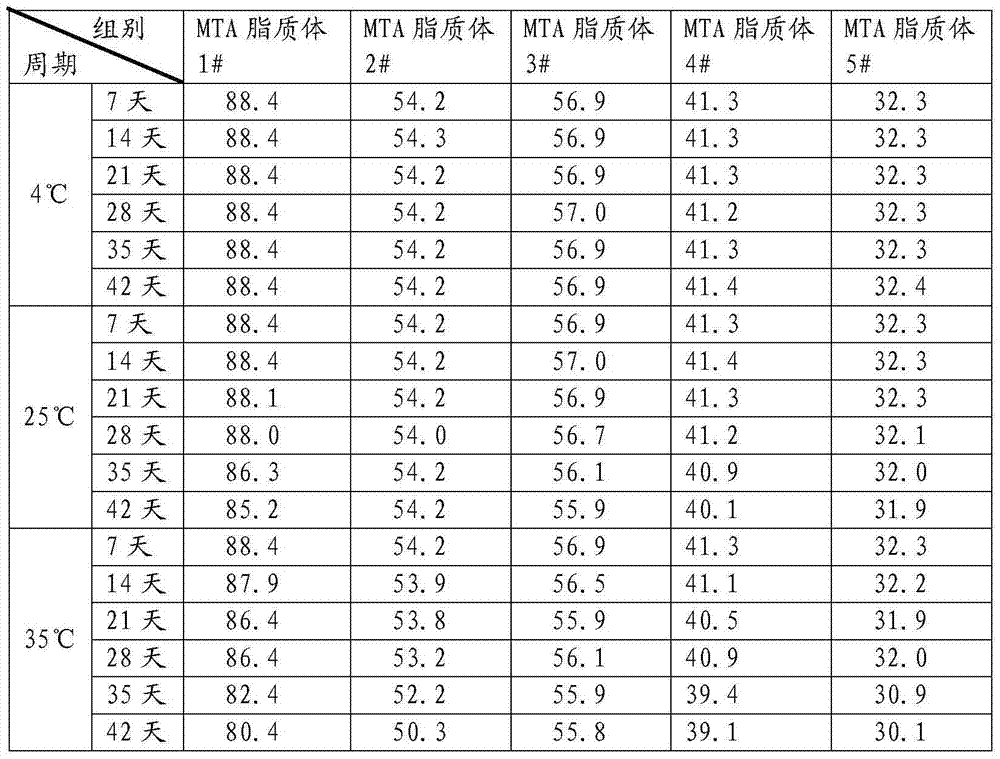
A kind of pemetrexed liposome and preparation method thereof
A technology of metrexed lipid and pemetrexed, applied in the field of pemetrexed liposome and its preparation, can solve the problems of increased impurity content of pemetrexed preparations, poor stability of pemetrexed, and poor absorption in vivo And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment one, MTA liposome 1#
[0047] Put 37mg of liposome mixture (HSPC / chol / TPGS mass ratio is 47.4 / 42.6 / 10) in ep tube and add 0.5ml chloroform to dissolve; the mixture is placed in a rotary evaporator, at room temperature, evaporated under reduced pressure to form an organic film Add 5ml of calcium acetate aqueous solution for hydration, rotary steaming 30min, remove residual organic solvent; the liposome ice bath ultrasonic 5min that forms; Get above-mentioned liposome solution and join in the dialysis bag that molecular weight cut off is 1000, dialysate ( The volume of NS solution) is 1L, and the dialysis time is 6-8 hours. The liquid (1L*2) is changed twice to form a calcium acetate gradient and increase the monitoring of pH (pH=4.5-5.5).
[0048] The liposome solution after dialysis was taken, and the pre-prepared 5 mg / ml pemetrexed solution (dissolved in MilliQ water) was added to the final concentration of 1 mg / ml, and the solution was bathed in water at 60...
Embodiment 2
[0049] Embodiment two, MTA liposome 2#
[0050]Put 30mg of liposome mixture (HSPC / chol / TPGS mass ratio is 50 / 45 / 5) in ep tube and add 0.5ml chloroform to dissolve; the mixture is placed in a rotary evaporator, at room temperature, evaporated under reduced pressure to form an organic film Add 5ml of calcium acetate aqueous solution for hydration, rotary steaming 20min, remove residual organic solvent; the liposome ice bath ultrasonic 10min that forms; Get above-mentioned liposome solution and join in the dialysis bag that molecular weight cut off is 1000, dialysate ( The volume of NS solution) is 1L, and the dialysis time is 6-8 hours. The liquid (1L*2) is changed twice to form a calcium acetate gradient and increase the monitoring of pH (pH=4.5-5.5).
[0051] The liposome solution after dialysis was taken, and the pre-prepared 10 mg / ml pemetrexed solution (dissolved in MilliQ water) was added to the final concentration of 2 mg / ml. During the drug loading process, the solution ...
Embodiment 3
[0052] Embodiment three, MTA liposome 3#
[0053] Put 40 mg of liposome mixture (HSPC / chol / TPGS mass ratio is 44.7 / 40.3 / 15) into an ep tube and add 1 ml of chloroform to dissolve; the mixture is placed in a rotary evaporator, at room temperature, and evaporated under reduced pressure to form an organic film; Add 10ml of calcium acetate aqueous solution for hydration, rotary steam for 60min, remove residual organic solvent; sonicate the formed liposome in an ice bath for 10min; take the above liposome solution and add it to a dialysis bag with a molecular weight cut-off of 1000, dialysate (NS The volume of solution) is 1L, and the dialysis time is 6-8 hours. The liquid (1L*2) is changed twice to form a calcium acetate gradient and increase the monitoring of pH (pH=4.5-5.5).
[0054] The liposome solution after dialysis was taken, and the pre-prepared 4 mg / ml pemetrexed solution (dissolved in MilliQ water) was added to the final concentration of 2 mg / ml. During the drug loading ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com