External preparation for treating facial seborrheic dermatitis
A technology for seborrheic dermatitis and external preparations, applied in the directions of medical preparations containing active ingredients, anti-inflammatory agents, topical antibacterial agents, etc., can solve the problems of low effective rate, easy recurrence of symptoms, etc. Inflammatory response and the effect of repairing epidermal barrier function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
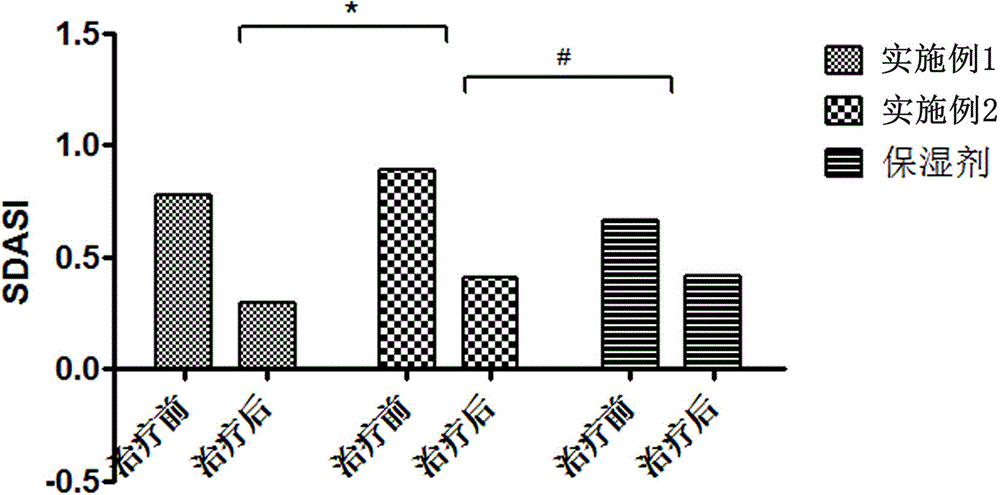
Image
Examples
Embodiment 1
[0020] Example 1: Add 6.2g glycerol and 10g triethanolamine into boiled 55ml distilled water, stir to dissolve, cool and keep the temperature at 80-85°C as the water phase; add 12.4g stearic acid, 12.4g castor oil and Heat and melt 12.4g of liquid paraffin, keep the temperature at 80-85°C, as the oil phase; add the above-mentioned oil phase to the above-mentioned water phase under slow stirring to complete the matrix preparation; when the matrix is cooled to 50-60°C, add the drug Add 0.05g of chlorhexidine acetate, 0.05g of ketoconazole, 0.05g of ceramide, 8g of glycerol, and 0.8g of glycyrrhizin into the aforementioned base, stir evenly in the same direction, and cool to room temperature.
Embodiment 2
[0021] Embodiment 2: 6.2g glycerin and 10g triethanolamine are added into boiled 55ml distilled water, stir to dissolve, cool and keep the temperature at 80-85°C, as the water phase; 12.4g stearic acid, 12.4g castor oil and Heat and melt 12.4g of liquid paraffin, keep the temperature at 80-85°C, and use it as the oil phase; add the aforementioned water phase to the aforementioned oil phase under slow stirring to complete the matrix preparation; when the matrix is cooled to 50-60°C, add the drug Add 0.08g of chlorhexidine acetate, 0.1g of ketoconazole, 0.1g of ceramide, 10g of glycerol, and 1g of glycyrrhizin into the aforementioned base, stir evenly in the same direction, and cool to room temperature.
Embodiment 3
[0022] Example 3: Take 10 g of vaseline as the base, add the medicinal ingredients 0.1 g of chlorhexidine acetate, 0.15 g of ketoconazole, 0.15 g of ceramide, 12 g of glycerol, and 1.2 g of glycyrrhizin, and stir to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com