Biodegradable and absorbable hemostasis composition
A technology of biodegradation and composition, applied in the direction of drug combination, absorbent pads, blood diseases, etc., can solve the problem of not seeing hyaluronic acid, etc., and achieve the effect of promoting the formation of blood clots
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
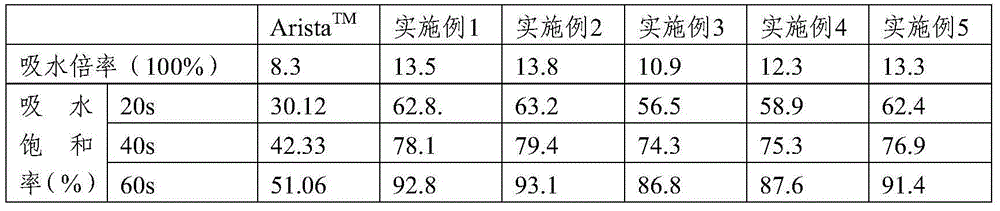
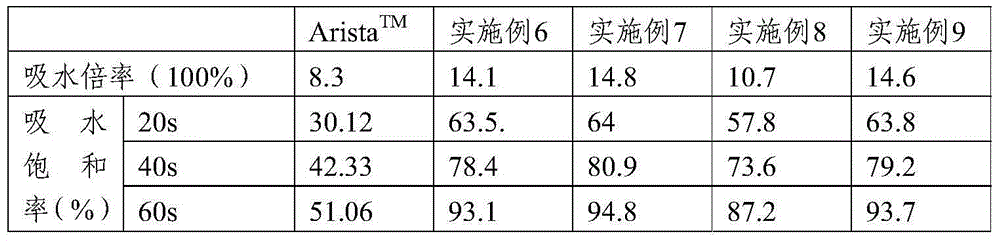
[0065] Example 1: Hemostatic Composition
[0066] 1. Composition: 5g sodium hyaluronate, 10g carboxymethyl chitosan and 85g carboxymethyl starch.
[0067] 2. Preparation method:
[0068] Take carboxymethyl starch, sodium hyaluronate and carboxymethyl chitosan according to the proportioning ratio, then dissolve it in 10L water, stir well (concentration g / ml is respectively 0.85%, 0.05%, 0.1%), and use Freeze-dry in a vacuum freeze dryer for 24 hours (set freezing temperature -15°C, vacuum degree o.8pa), grind the freeze-dried composite film into powder and pass through a 35-mesh sieve, and then sterilize by irradiation to obtain sterile biodegradable hemostatic powder.
Embodiment 2
[0069] Example 2: Hemostatic Composition
[0070] 1. Composition: 5g sodium hyaluronate, 5g carboxymethyl chitosan and 90g carboxymethyl starch.
[0071] 2. Preparation method:
[0072] Take carboxymethyl starch, sodium hyaluronate and carboxymethyl chitosan according to the proportion, dissolve them in 10L water, stir evenly (concentration g / ml is respectively 0.05%, 0.05%, 0.9%), and freeze-dry in vacuum Machine freeze-drying for 24 hours (set freezing temperature -20°C, vacuum degree o.5pa), grind the freeze-dried composite film into powder, pass through a 35-mesh sieve, and then sterilize by irradiation to obtain a sterile biodegradable hemostatic powder .
Embodiment 3
[0073] Example 3: Hemostatic Composition
[0074] 1. Composition: 40g sodium hyaluronate and 60g carboxymethyl starch.
[0075] 2. Preparation method:
[0076] Take sodium hyaluronate and carboxymethyl starch according to the proportioning ratio, dissolve them in 10L water (concentration g / ml is 0.6%, 0.4% respectively), stir evenly, dry by spray drying method, and then irradiate and sterilize to obtain Bacterial biodegradable hemostatic powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com