Method for synthesizing metal cyanides by use of Fenton reagent
A cyanide and metal technology, applied in the chemical field, can solve the problems of difficult synthesis of multimetal cyanide, unstable product quality, light pollution, etc., and achieve the effect of energy saving and environmental protection of the reaction route, no light pollution, and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
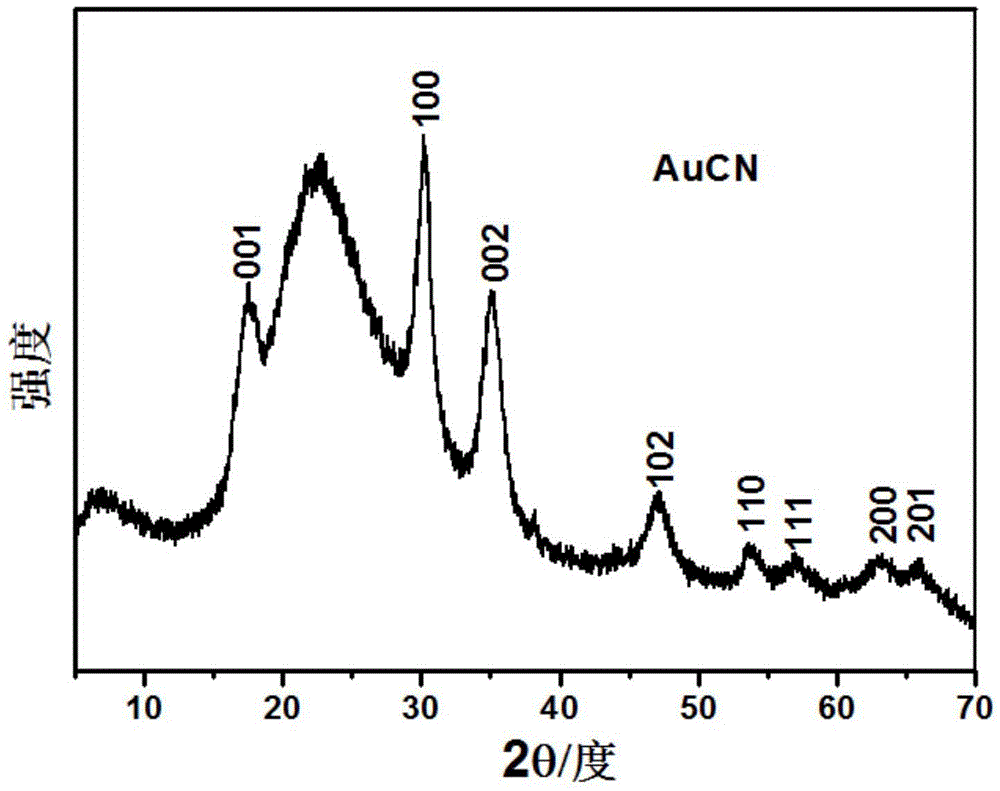
[0037] Mix 0.8 mg of nano-gold supported on silica, 2 mL of acetonitrile, 22 mg of ferrous sulfate heptahydrate, and 0.8 mmol of hydrogen peroxide, and stir at 30°C for 4 hours. The color of the solid gradually changes from red to bean paste green; the product is centrifuged, Silica-supported gold cyanide (AuCN) was obtained after drying treatment. figure 1 This is the XRD spectrum of the gold cyanide, in which it can be seen that the product is pure gold cyanide, the gold is completely cyanided, and no ferricyanide is generated.
Embodiment 2
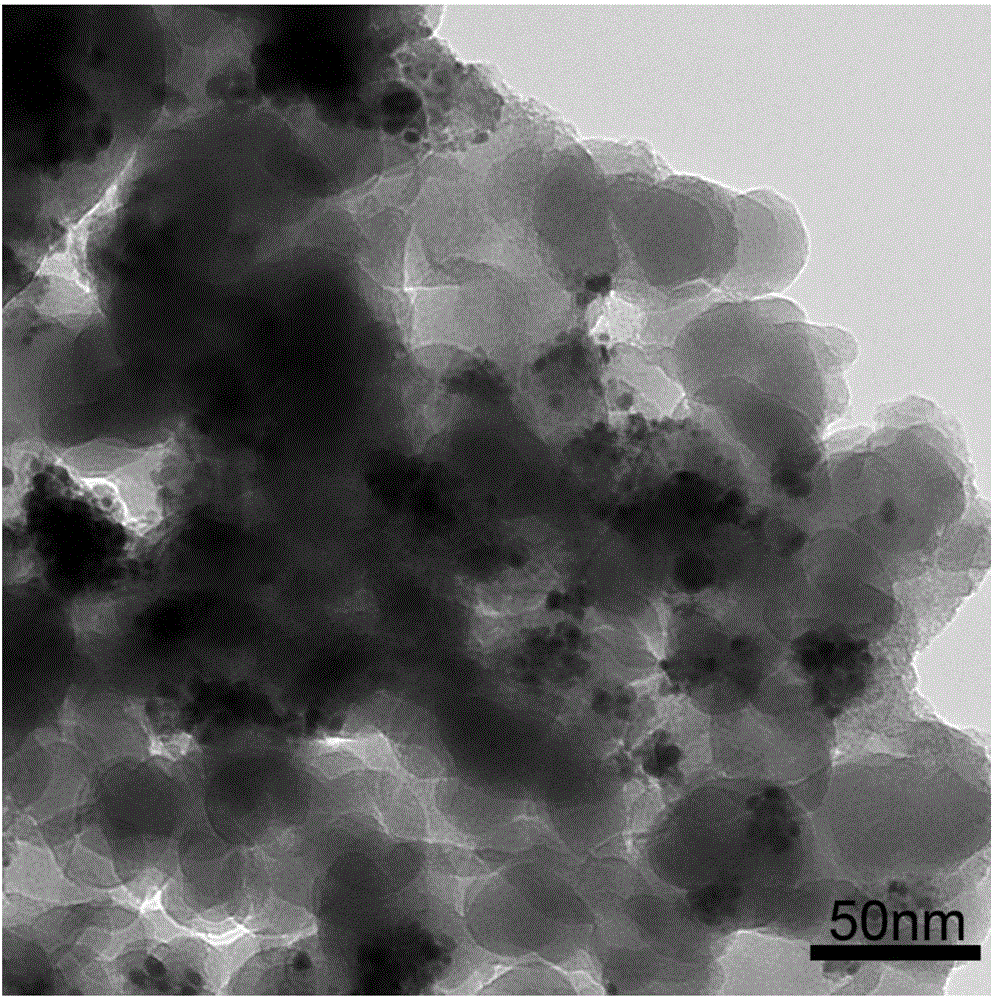
[0045] Mix 2mg of nano-gold loaded on silica, 3mL of acetonitrile, 3mg of ferrous sulfate heptahydrate, and 1mmol of hydrogen peroxide, and stir at 70°C for 30 minutes, the color of the solid gradually changes from red to bean paste green; the product is centrifuged and dried Finally, silica-supported gold cyanide (AuCN) was obtained. image 3 is a transmission electron micrograph of gold cyanide (AuCN). No ferricyanide was detected.
Embodiment 3
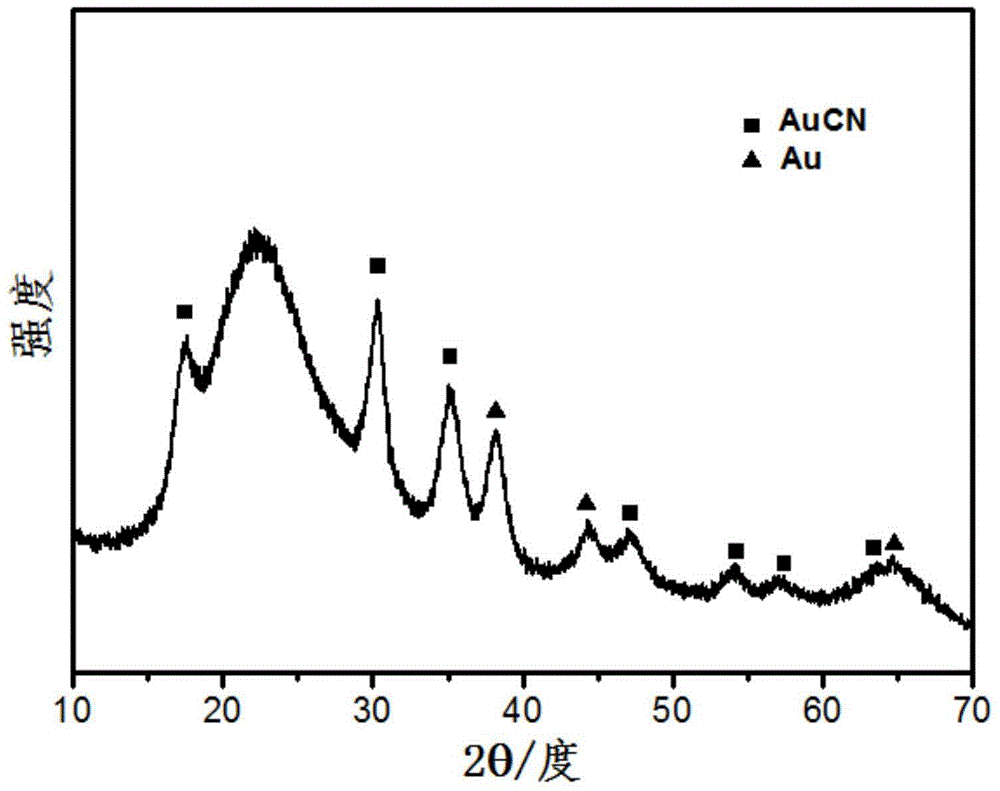
[0047] Mix 10 mg of nano-gold loaded on silica, 1.3 mL of propionitrile, 30 mg of ferrous sulfate heptahydrate, and 21 mmol of hydrogen peroxide, and stir at 10°C for 48 hours. The color of the solid gradually changes from red to bean paste green; the product is centrifuged, Silica-supported gold cyanide (AuCN) was obtained after drying treatment. Figure 4 It is the XPS spectrum of gold cyanide (AuCN), showing Au4f 5 / 2 and Au4f 7 / 2 The peak position of corresponds to that of Au(I). No ferricyanide was detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com