Method for preparing environment-sensitive cyclodextrin derivative hydrogel
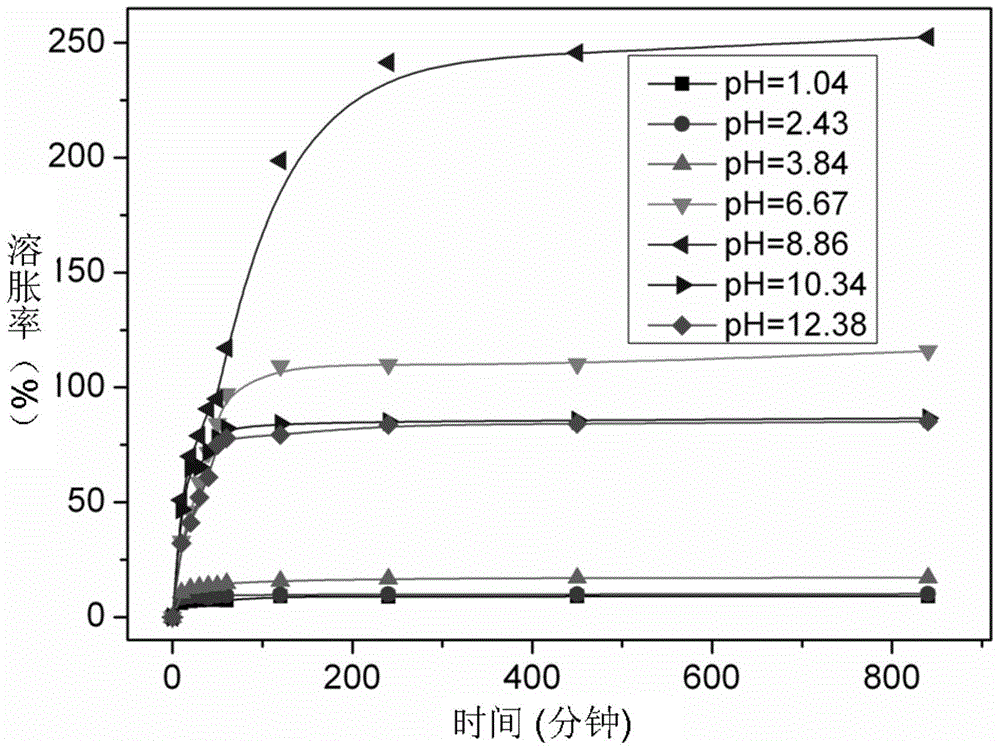
An environment-sensitive, cyclodextrin technology, applied in the direction of inactive components of polymer compounds, can solve problems such as slow speed, achieve good swelling, achieve temperature-sensitive type, and achieve the effect of drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
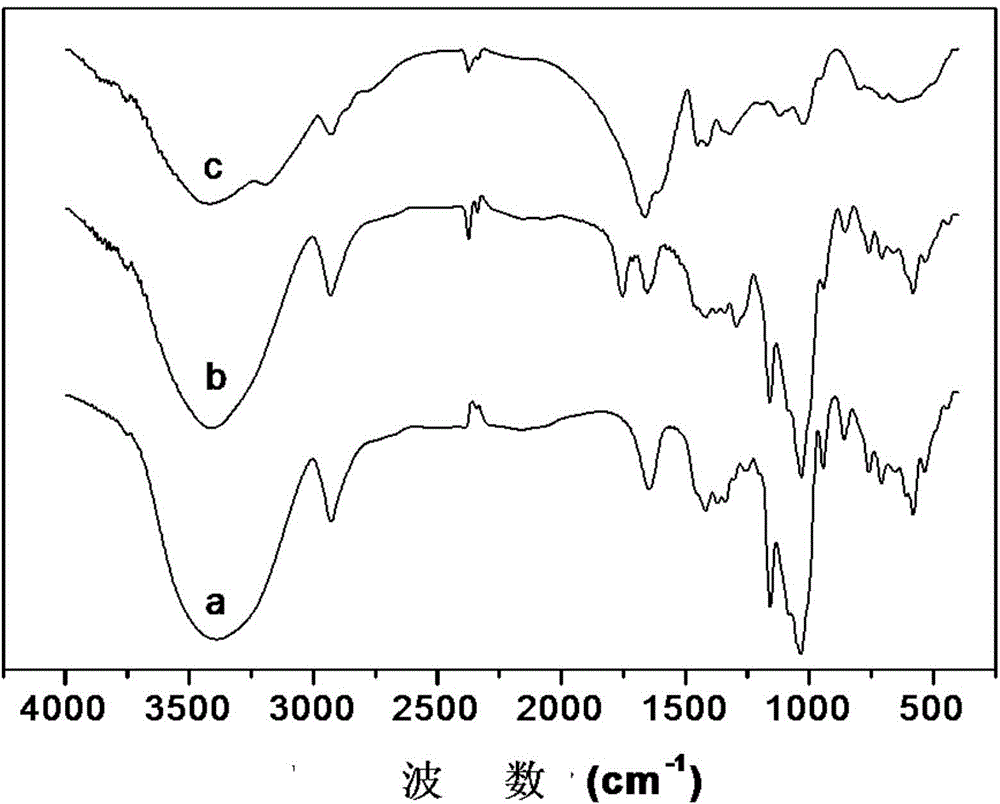
[0029] Weigh 1.135g (0.001mol) of β-cyclodextrin (β-CD) and dissolve it in 12mL N,N-dimethylformamide (DMF) and stir until dissolved, then add 0.172g ( 0.002mol) of methacrylic acid (MAA) and 0.618g (0.003mol) of 1,2-dicyclohexylcarbonyldiimide (DCC), stirred for 30min, and reacted at 35°C for 5h. After the reaction, the solid was removed by vacuum filtration, the filtrate was precipitated with isopropanol, and the filter cake was a crude product, and the crude product was redissolved with 50% ethanol solution. After filtration, the filtrate was precipitated with isopropanol again, filtered, filtered The cake was dried to give the product methacrylate-β-cyclodextrin (MAA-β-CD) as a white powder solid.
[0030] Dissolve 0.8g of cross-linking agent N,N'-methylenebisisoacrylamide (BIS) in 20mL of dimethyl sulfoxide (DMSO), stir at 0°C for 3 hours, then dissolve 3.2g of monomer MAA Add 0.8g of MAA-β-CD into the reaction system, and at the same time add 0.2g of acrylamide (MBAA) to ...
Embodiment 2
[0036] Weigh 1.135g (0.001mol) of β-cyclodextrin (β-CD) and dissolve it in 12mL of N,N-dimethylformamide (DMF) and stir until dissolved, then add 0.002mol of formamide under ice bath conditions at 5°C Acrylic acid (MAA) and 0.003mol of 1,2-dicyclohexylcarbonyldiimide (DCC), stirred for 30min, and placed at 35°C for 5h. After the reaction, the solid was removed by vacuum filtration, the filtrate was precipitated with isopropanol to precipitate the crude product, and the crude product was re-dissolved with 50% ethanol solution, after filtration, the filtrate was precipitated with isopropanol again, and vacuum-dried to obtain a white powder solid product Methacrylate-β-cyclodextrin (MAA-β-CD).
[0037] Dissolve 0.8 g of cross-linking agent N,N'-methylenebisisoacrylamide (BIS) in 20 mL of distilled water and dimethyl sulfoxide (DMSO) mixture (V DMSO :V H2O =3.50:0.5), after stirring at 0°C for 3 hours, add 3.2g of monomer MAA and 0.8g of MAA-β-CD into the reaction system, and ad...
Embodiment 3
[0039] Weigh 1.135g (0.001mol) of β-cyclodextrin (β-CD) and dissolve it in 12mL of N,N-dimethylformamide (DMF) and stir until dissolved, then add 0.002mol of formamide under ice bath conditions at 5°C Acrylic acid (MAA) and 0.003mol of 1,2-dicyclohexylcarbonyldiimide (DCC), stirred for 30min, and placed at 35°C for 5h. After the reaction was finished, the solid was removed by vacuum filtration, and the filtrate was precipitated with isopropanol to precipitate the crude product, and the crude product was redissolved with 50% ethanol solution, and after filtration, the precipitate was precipitated with isopropanol again, and vacuum-dried to obtain the white powder solid product A Acrylate-β-cyclodextrin (MAA-β-CD).
[0040] Dissolve 0.8 g of cross-linking agent N,N'-methylenebisisoacrylamide (BIS) in 20 mL of distilled water and dimethyl sulfoxide (DMSO) mixture (V DMSO :V H2O =3.0:1.0), after stirring at 0°C for 3 hours, add 3.2g of monomer MAA and 0.8g of MAA-β-CD into the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com