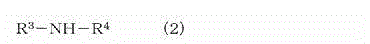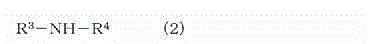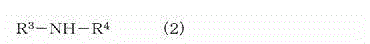Crosslinkable rubber composition and crosslinked rubber
A rubber composition and cross-linking technology, applied in the field of rubber cross-linked products and cross-linked rubber compositions, to achieve excellent compression set resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0101] There are no particular limitations on the preparation method of the crosslinkable rubber composition of the present invention, and examples include mixing a carboxyl group-containing nitrile rubber (A), a polyamine crosslinking agent (B) and a basic crosslinking accelerator (C). method. It should be noted that in this case, it is preferable that the basic crosslinking accelerator (C) forms a salt with the above-mentioned alkylene glycol together with an inorganic acid and / or an organic acid as needed, and the salt is mixed with carbon It is used in a state where alkyl alcohols of the number 5 to 20 are mixed.
[0102] There is no particular limitation on the mixing method, but mixing in a non-aqueous system (dry mixing) is preferred.
[0103] Specifically, it can be prepared as follows: when mixing carboxyl group-containing nitrile rubber (A), polyamine crosslinking agent (B) and basic crosslinking accelerator (C), add polyamine crosslinking agent (B), Each component...
Embodiment
[0115] Hereinafter, the present invention will be specifically described with reference to Examples and Comparative Examples. Hereinafter, "parts" are based on weight unless otherwise specified. In addition, the test and evaluation were performed as follows.
[0116] iodine value
[0117] The iodine value of the nitrile rubber is measured in accordance with JIS K 6235.
[0118] Carboxyl content
[0119] Add 100ml of 2-butanone to 0.2g of nitrile rubber with a square of 2mm, and stir for 16 hours, then add 20ml of ethanol and 10ml of water, and use a 0.02N aqueous ethanol solution of potassium hydroxide while stirring. At room temperature, use In the titration using thymolphthalein as an indicator, it was determined as the number of moles of carboxyl groups relative to 100 g of nitrile rubber (the unit is ephr).
[0120] Composition of nitrile rubber
[0121] The content ratio of each monomer unit constituting the hydrogenated carboxyl group-containing nitrile rubber...
manufacture example 1
[0134] Manufacturing example 1 (manufacture of carboxyl group-containing nitrile rubber (a1))
[0135] Into the reactor, 220 parts of ion-exchanged water, 5 parts of sodium dodecylbenzenesulfonate aqueous solution with a concentration of 10%, 37 parts of acrylonitrile, 4 parts of mono-n-butyl maleate, and tert-dodecyl After replacing 0.75 parts of mercaptan (molecular weight modifier) with nitrogen gas inside, 57 parts of 1,3-butadiene was charged. Then, the reactor was kept at 10° C., 0.06 parts of cumene hydroperoxide (polymerization initiator) was charged, and the polymerization reaction was continued while stirring. When the polymerization conversion rate became 40% and 60%, each 1 part of mono-n-butyl maleate was added, and when the polymerization conversion ratio became 85%, 0.1 part of a hydroquinone aqueous solution (polymerization terminator) with a concentration of 10% by weight was added to terminate the polymerization reaction. Next, residual monomers were rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com