High-throughput drug screening model using human pregnane X receptor (hPXR)-mediated dual-luciferase reporter gene technique
A luciferase and reporter gene technology, applied in the fields of molecular biology and pharmacology, can solve problems affecting the expression and adverse effects of metabolic enzymes and transporters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Routine culture of HEK293T cells
[0027] HEK293T cells were grown in DMEM high-glucose medium containing 10% fetal bovine serum, 100 U / mL penicillin and streptomycin, and adhered to the wall at 37°C, 5% CO2, and saturated humidity. When the cells grow to 80% ~ 90% confluent, they are digested with 0.25% trypsin digestion solution, the digestion time is 1 ~ 2min, and passaged at 1:3.
Embodiment 2
[0029] Plasmid extraction
[0030] 1) Pick and inoculate the Escherichia coli containing the plasmid in 30 mL LB liquid medium for culture, shake at 37 °C, 260 rpm / min, and shake for 12-16 h to obtain a small shake liquid. Collect the bacteria by centrifuging at 3500–5000 g for 30 min at room temperature.
[0031] 2) Discard the medium supernatant. Add 250 μL Solution I / RNaseA resuspension solution and vortex to completely suspend the cells.
[0032] 3) Add 250 μL of Solution II lysate to the resuspension mixture, immediately invert and mix 4-6 times, then place the mixture at room temperature for 2 minutes to increase the yield, until the solution is clear. Avoid vigorous mixing of the lysate and the lysis reaction should not exceed 5 min.
[0033] 4) Add 300 μL Solution III neutralizing solution, and gently invert the centrifuge tube several times until a white flocculent precipitate is formed. high speed centrifugation
[0034] 5) Carefully transfer the supernatant to ...
Embodiment 3
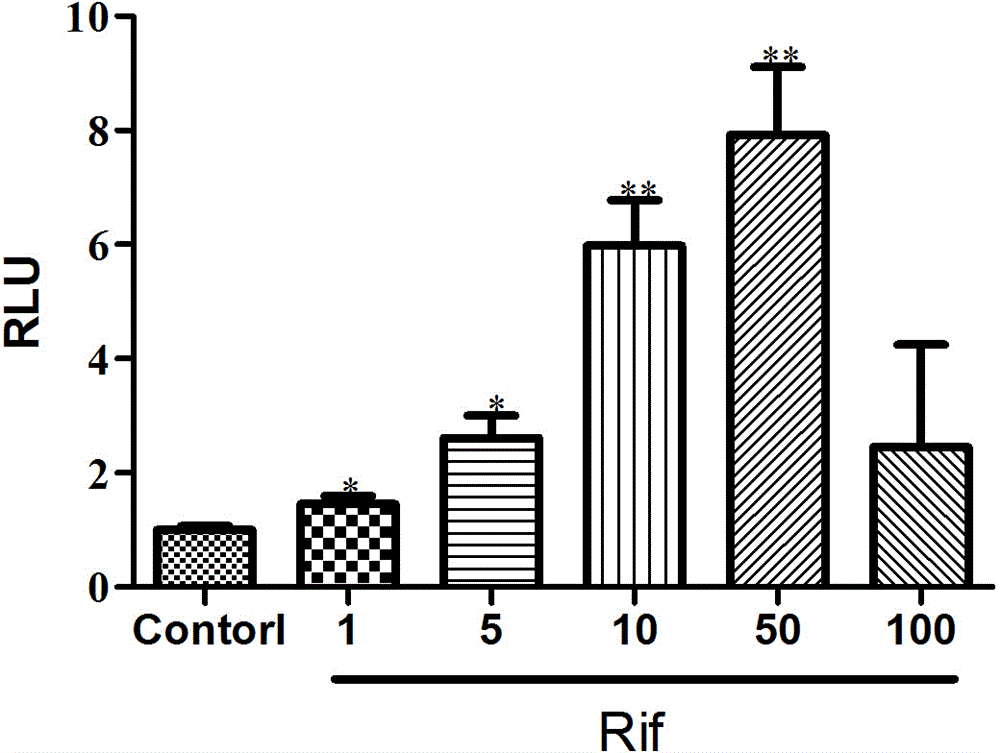
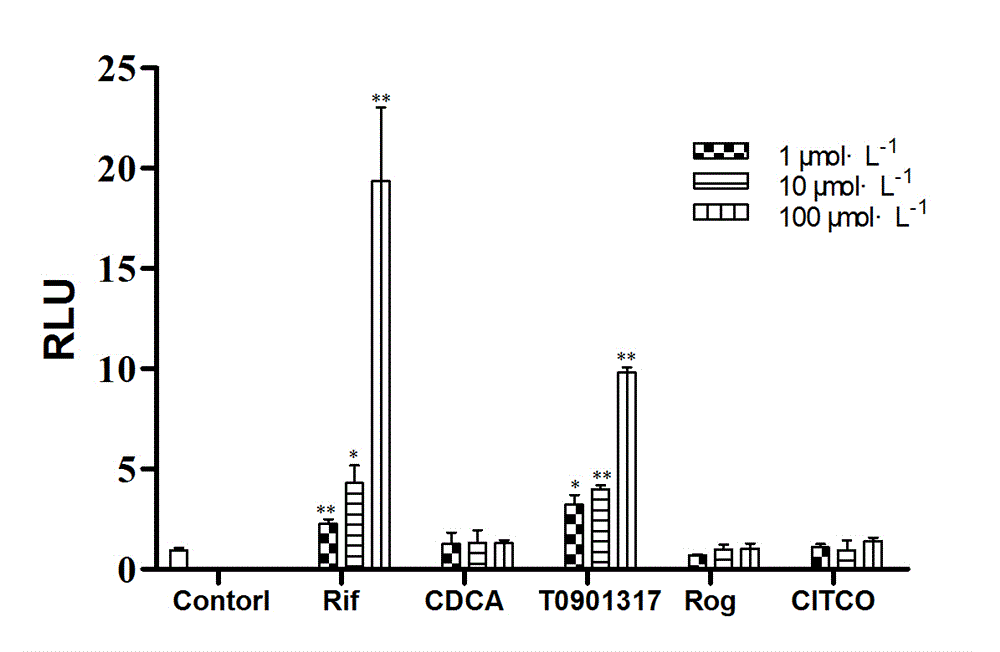
[0042] According to the optimal transfection conditions obtained, HEK293 cells transiently transfected with PXR expression plasmids, reporter gene plasmids, and internal reference plasmids were given corresponding positive drugs, and the dual luciferase activity was detected after drug stimulation for 24 h. The results were as follows: figure 1 As shown, the relative fluorescence intensity increases dose-dependently with the positive drug, and the relative fluorescence intensity of 50 μM Rif can reach about 8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com