A kind of high chemical stability multilayer composite proton exchange membrane and its preparation method and application
A proton exchange membrane and chemical stability technology, applied in the field of high chemical stability multi-layer composite proton exchange membrane and its preparation, can solve the problems of unsuitable proton exchange membrane fuel cell, lack of protection, etc., to ensure stability and performance consistency, the effect of enhancing stability and consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
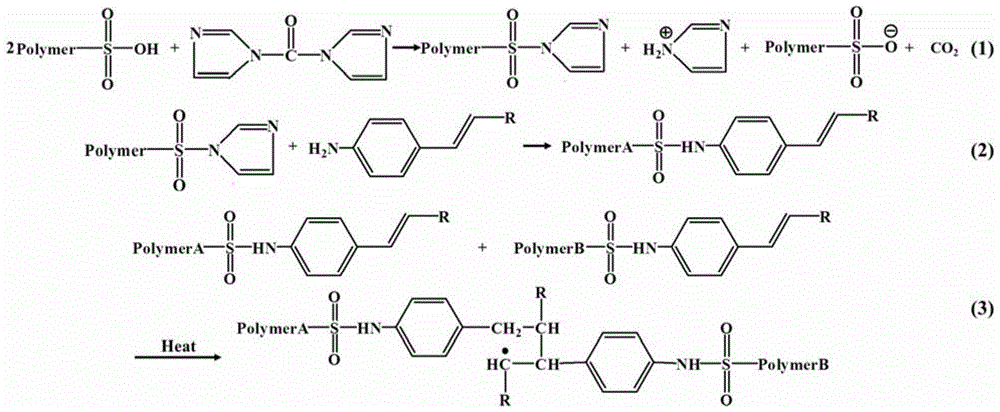
[0078] (1) Take 0.6g sulfonated polyether ether ketone (SPEEK obtained by sulfonation reaction of PEEK whose model is Vitrex 450PF, IEC=1.95mmol g -1 ) was dissolved in 4mL DMSO, added 0.04g CDI, reacted at 50°C for 2h, added 0.032g 4-aminostyrene (VA), reacted at room temperature for 8h, and prepared a modified SPEEK casting solution;
[0079] (2) Lay the above-mentioned modified SPEEK casting film on a glass plate, and bake at 60°C for 8 hours to obtain a base film with a thickness of 79 μm;
[0080] (3) Dissolve 0.5g Nafion resin in 4mL DMSO, add 0.02g CDI, react at 50°C for 2h, add 0.017g 4-aminostyrene, react at room temperature for 8h, and make a modified Nafion casting solution;
[0081] (4) Coating modified Nafion on both sides of the base film prepared in the above step (2) by means of dipping-lifting.
[0082](5) Spread the multilayer composite membrane on a glass plate, bake it at 60°C for 8 hours, and finally place it in a vacuum drying oven at 150°C for 3 hours t...
Embodiment 2
[0087] In the same way as in Example 1, the difference is that the non-fluorine sulfonic acid polymer used is sulfonated polyethersulfone (SPES obtained by the PES of SolvayVeradel-3000P through sulfonation reaction, IEC=2.64mmol g -1 ).
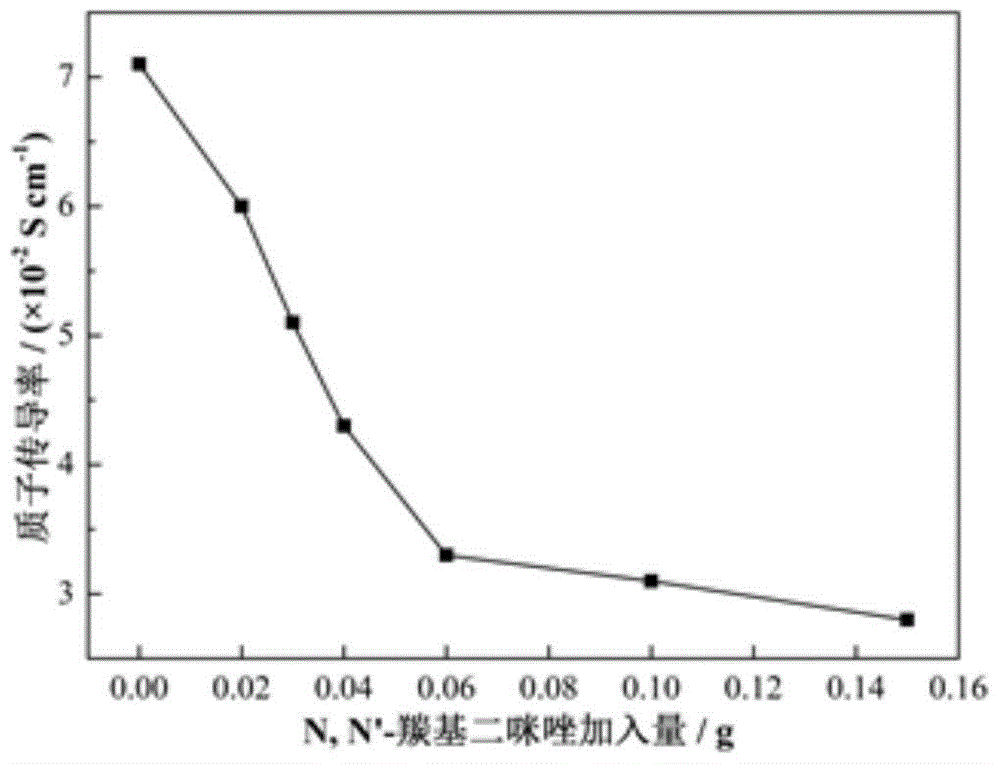
[0088] The multilayer composite proton exchange membrane prepared by the above method was subjected to an accelerated oxidation test at 80° C. in Fenton reagent, and the performance was similar to that of the multilayer composite proton exchange membrane obtained in Example 1.
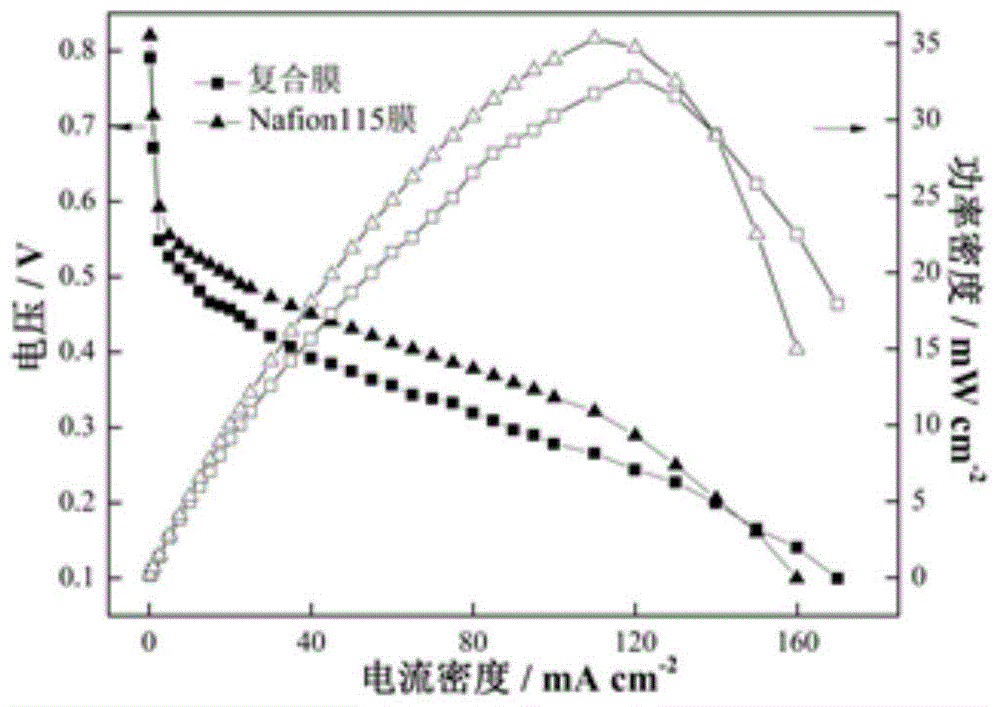
[0089] The multilayer composite proton exchange membrane prepared by the above method is assembled into a direct methanol fuel cell, and the effective area of the membrane electrode is 4cm -2 , the catalyst loading of positive and negative electrodes is 4 mg cm -2 , using a methanol solution with a concentration of 3M. The measured polarization curve and power density curve of the multilayer composite film are close to those of Example 1.
[0090] The multi-laye...
Embodiment 3
[0092] In the same way as in Example 1, the difference is that the non-fluorine sulfonic acid polymer used is sulfonated polysulfone (the SPSU obtained by the PSU of Solvay P3500 by sulfonation reaction, IEC=2.64mmol g -1 ).
[0093] The multilayer composite proton exchange membrane prepared by the above method was subjected to an accelerated oxidation test at 80° C. in Fenton reagent, and the performance was similar to that of the multilayer composite proton exchange membrane obtained in Example 1.
[0094] The multilayer composite proton exchange membrane prepared by the above method is assembled into a direct methanol fuel cell, and the effective area of the membrane electrode is 4cm -2 , the catalyst loading of positive and negative electrodes is 4 mg cm -2 , using a methanol solution with a concentration of 3M. The measured polarization curve and power density curve of the multilayer composite film are close to those of Example 1.
[0095] The multi-layer composite p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com