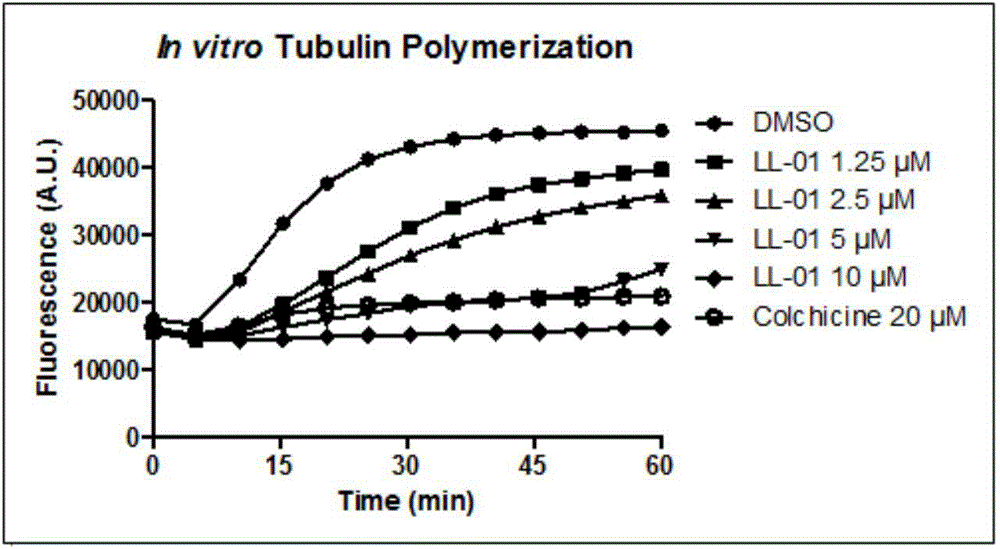
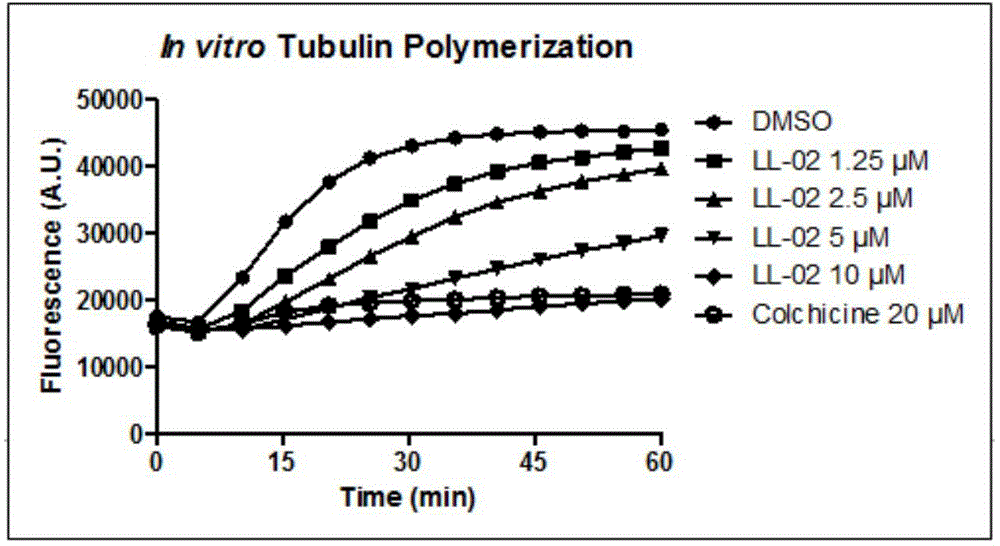
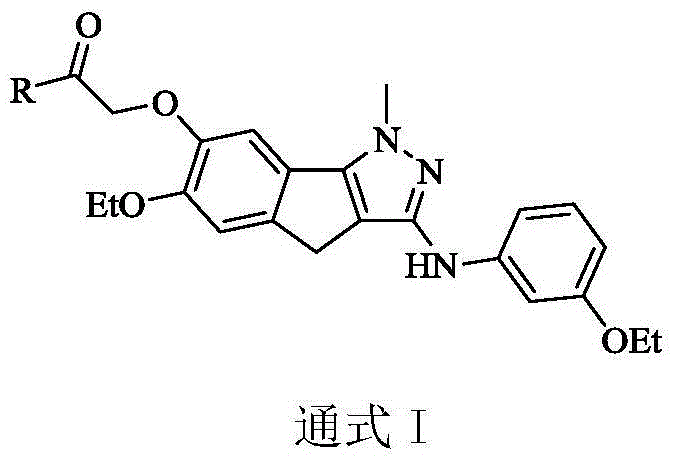
Indeno pyrazole micromolecular tubulin inhibitor and preparation method and application thereof
A technology of tubulin inhibition and indenopyrazoles, which is applied in the chemical field, can solve the problems of drug resistance, high toxicity and side effects, and difficult synthesis, and achieve novel structure, reduce toxicity and side effects, and enhance specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of structural formula 1 compound
[0035] (1) Dissolve 5-ethoxy-6-hydroxy-1-indanone (Formula 1) (3.20g) in N,N-dimethylformamide (25mL), add imidazole (1.70g), and stir After 5 min, tert-butyldimethylsilyl chloride (3.76 g) was added and stirred for 1 h. Add a citric acid aqueous solution (100mL) with a mass fraction of 10%, let it cool in a refrigerator, filter with suction, and dry in vacuo to obtain 5-ethoxy-6-tert-butyldimethylsiloxy-1-indanone ( Formula 2) 4.65g, the yield is 91.4%. Melting point: 142–143°C. ESI-MS m / z 307.5[MH] + .
[0036](2) Dissolve 5-ethoxy-6-tert-butyldimethylsiloxy-1-indanone (Formula 2) (4.65g) in anhydrous tetrahydrofuran (60mL), cool to -78°C, Add 1M tetrahydrofuran solution (18.2mL) of lithium bis(trimethylsilyl)amide dropwise, stir for 2h, then raise the temperature to -45°C within 45min, add 3-ethoxylate dissolved in anhydrous tetrahydrofuran (15mL) phenyl phenyl isothiocyanate (3.17g), room tempera...
Embodiment 2
[0041] Embodiment 2: the preparation of structural formula 2 compound
[0042] (1) Dissolve 5-ethoxy-6-hydroxy-1-indanone (Formula 1) (3.20g) in N,N-dimethylformamide (25mL), add imidazole (1.70g), and stir After 5 min, tert-butyldimethylsilyl chloride (3.76 g) was added and stirred for 1 h. Add a citric acid aqueous solution (100mL) with a mass fraction of 10%, let it cool in a refrigerator, filter with suction, and dry in vacuo to obtain 5-ethoxy-6-tert-butyldimethylsiloxy-1-indanone ( Formula 2) 4.65g, the yield is 91.4%. Melting point: 142–143°C. ESI-MS m / z 307.5[MH] + .
[0043] (2) Dissolve 5-ethoxy-6-tert-butyldimethylsiloxy-1-indanone (Formula 2) (4.65g) in anhydrous tetrahydrofuran (60mL), cool to -78°C, Add 1M tetrahydrofuran solution (18.2mL) of lithium bis(trimethylsilyl)amide dropwise, stir for 2h, then raise the temperature to -45°C within 45min, add 3-ethoxylate dissolved in anhydrous tetrahydrofuran (15mL) phenyl phenyl isothiocyanate (3.17g), room temper...
Embodiment 3
[0048] Example 3: Anti-proliferation test
[0049] 1. Test method:
[0050] Human liver cancer HepG2 cells, human prostate cancer PC3 cells, human cervical cancer HeLa cells, human breast cancer MCF-7 cells and human leukemia K562 cells were given different concentrations of the compound of formula 1 (prepared in Example 1) and the compound of formula 2 ( Embodiment 2 preparation), put 37 ℃, 5%CO 2 The incubator was incubated for 72 hours, and the inhibitory rate of the compound on tumor cells was determined by the tetramethylazolate (MTT) colorimetric method, and the results are shown in Table 1.
[0051] 2. Test results:
[0052] Table 1 The compound of the present invention is to the inhibitory rate of different tumor cells
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com