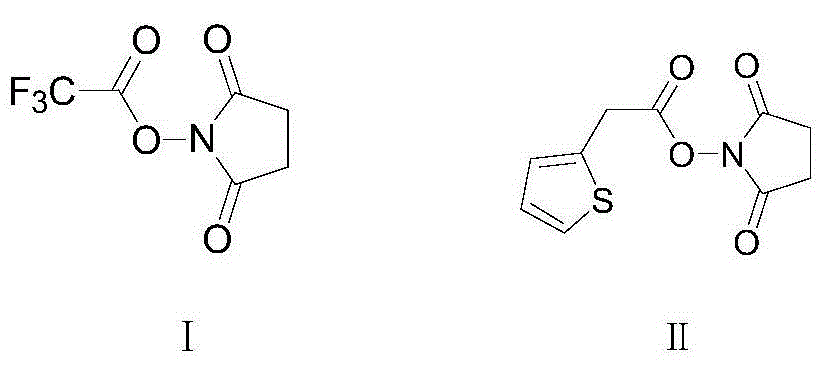
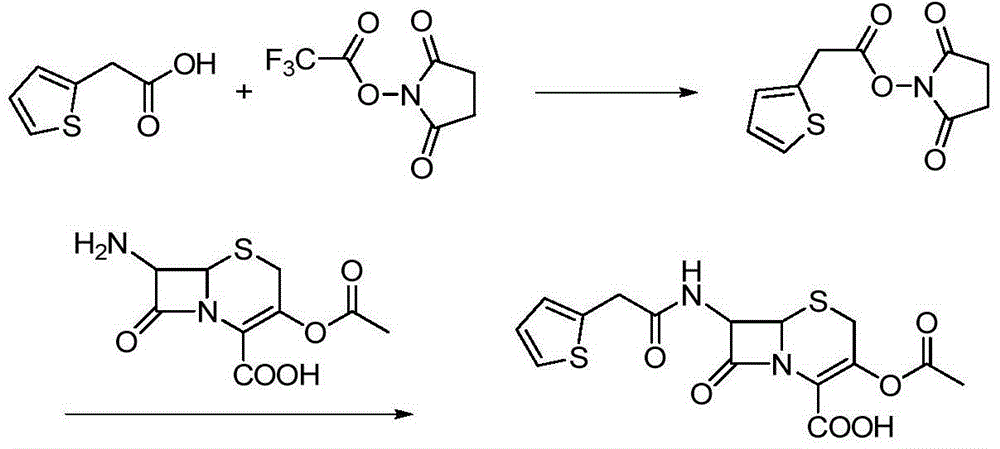
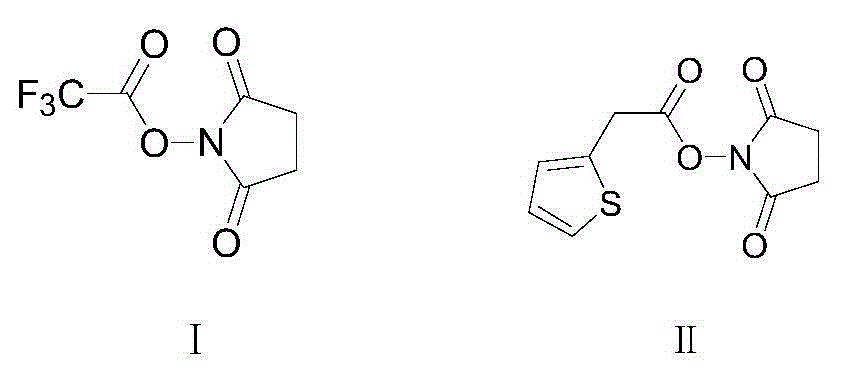
Preparation method of cephalotin acid
A technology of cephalothinic acid and thiopheneacetic acid, which is applied in the direction of organic chemistry, can solve the problems of strong irritating odor, unfriendly environment, instability, etc., and achieve the effects of being conducive to industrial production, simplifying process operation, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 2-thiopheneacetic acid 71g (0.5mol) in the three-necked flask of 2000mL, organic solvent dichloromethane 600mL, then add 90mL triethylamine and trifluoroacetic acid succinimide ester 127g (0.6mol), then control the temperature at 15 Under the condition of ℃~20℃, keep stirring and react for 3 hours. After the reaction is completed, filter with suction to obtain the reaction solution containing active ester, and slowly add the reaction solution containing active ester dropwise to the pre-prepared 1000mL di In methyl chloride solvent, and control the temperature under the condition of 15 ℃ ~ 20 ℃ to carry out the condensation reaction for 1 hour, after the condensation reaction is completed, add 10wt% hydrochloric acid solution dropwise to the reaction solution to adjust the pH value to 1.5 ~ 2.0, then statically Place and separate layers, collect the organic phase, quickly add the collected organic phase to 1000mL sodium bicarbonate aqueous solution, and adjust the pH ...
Embodiment 2
[0023] Add 2-thiopheneacetic acid 71g (0.5mol) in the there-necked flask of 2000mL, organic solvent chloroform 500mL and DMF100mL, add 90mL triethylamine and trifluoroacetic acid succinimide ester 106g (0.5mol) again, then control temperature at 10 Under the condition of ℃~20℃, keep stirring and react for 4 hours. After the reaction is completed, filter with suction to obtain the reaction solution containing active ester. Slowly add the active ester solution dropwise to the pre-prepared 1000mL chloroform solvent containing 109g 7-ACA. And control the temperature under the condition of 35 ℃ to carry out the condensation reaction for 1 hour. After the condensation reaction is completed, add 10 wt% hydrochloric acid solution dropwise to the reaction solution to adjust the pH value to 1.5-2.0. Then, let stand, separate layers, and collect the organic phase , quickly add the collected organic phase to 1000mL of sodium bicarbonate aqueous solution, and adjust the pH value to 6.5-7.0,...
Embodiment 3
[0025] Add 71g (0.5mol) of 2-thiopheneacetic acid in a 2000mL three-necked flask, 600mL of organic solvent ethylene glycol dimethyl ether, then add 80mL of morpholine and 212g (1.0mol) of succinimide trifluoroacetate, and then control the temperature Insulated and stirred for 4.5 hours under the condition of 10°C to 15°C, after the reaction was completed, suction filtered to obtain a reaction solution containing active ester, which was slowly added dropwise to the pre-prepared solution containing 136g (0.5mol) 7-ACA in 1000mL of ethylene glycol dimethyl ether solvent, and control the temperature at 15°C to 25°C for condensation reaction for 1 hour. After the condensation reaction is over, add 10wt% hydrochloric acid solution dropwise to the reaction solution to adjust the pH value to 1.5-2.0, then, stand still, separate layers, collect the organic phase, quickly add the collected organic phase to 1000mL sodium bicarbonate aqueous solution, and adjust the pH value to 6.5-7.0, st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com