A kind of metal iridium complex and its application
A technology of complexes and metal iridium, which is applied in the direction of indium organic compounds, platinum group organic compounds, and compounds containing group 8/9/10/18 elements of the periodic table, etc., to achieve large Stokes shift, good phosphorescence performance, The effect that is not easy to interfere with the background
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The synthesis steps of complex (I) are as follows:
[0016] Under nitrogen protection, 25 mg [(ppy) 2 IrCl] 2 and 10 mg of 2,3-bis(2-pyridyl)pyrazine (bppz) were dissolved in a solvent mixed with dichloromethane and methanol at a volume ratio of 1:1, heated to reflux under nitrogen for 12 hours, cooled to room temperature, and then The solvent was distilled off under reduced pressure, and the chromatographic column was used for purification (with CH 2 Cl 2 : MeOH=40:1 is the eluting system) to obtain an orange-red solid with a yield of about 80%:
[0017]
[0018] ESI-MS in positive ion mode (CH 2 Cl 2 ): m / z=735; H-NMR (400 MHz) δ: 8.73 (d, J =2.6Hz, 1H), 8.58(d, J =3.8Hz, 1H), 8.40(d, J =7.6Hz, 1H), 8.14(d, J =4.8Hz, 1H), 8.09(d, J =6.7Hz, 1H), 8.02(d, J =2.6Hz, 1H), 7.99 (d, J =4.7Hz, 1H), 7.93(t, J =7.7Hz, 3H), 7.82 (dd, J =16.8Hz, 8.4Hz, 3H), 7.71 (dd, J =7.1Hz, 4.5Hz, 2H), 7.54 (dd, J =7.5Hz, 4.8Hz, 1H), 7.42 (dd, J =14.3Hz, 7.3Hz, 2H), 7.22(t,...
Embodiment 2
[0020] The synthesis steps of complex (II) are as follows:
[0021] Under nitrogen protection, 25 mg [(ppy) 2 IrCl] 2 and 10 mg of 4,7-phenanthrolino-5,6:5',6'-pyraz-ine (ppz) ligand were dissolved in a solvent mixed with dichloromethane and methanol at a volume ratio of 1:1, and heated to reflux under nitrogen 12 hours, cooled to room temperature, then evaporated the solvent under reduced pressure, and purified by chromatographic column (using CH 2 Cl 2 : MeOH=40:1 is the eluting system) to obtain an orange-red solid with a yield of about 80%:
[0022]
[0023] ESI-MS in positive ion mode (CH 2 Cl 2 ): m / z=733; H-NMR (400 MHz) δ: 9.57 (1 H, d, J =8.4Hz), 9.50 (1 H, d, J =8.6Hz), 9.33 (2 H, d, J =3.5Hz), 8.49-8.42 (2 H, m), 8.19 (2 H, dd, J =8.3Hz, 4.0Hz), 8.16-8.08 (2 H, m), 7.92 (2 H, d, J =7.8Hz), 7.86 (2 H, t, J =6.3Hz), 7.69 (1H, d, J =6.0Hz), 7.57 (1 H, d, J =5.7Hz), 7.13 (2 H, t, J =7.6Hz), 7.00 (2 H, t, J =7.3Hz), 6.95 (2 H, s), 6.41 (2 H, dd, J =16H...
Embodiment 3
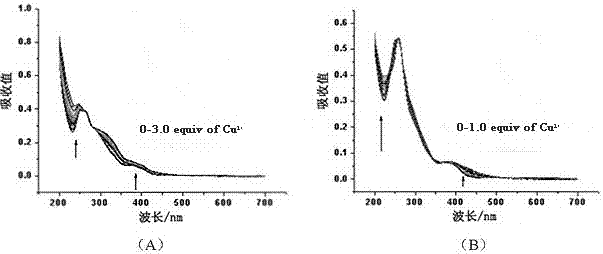
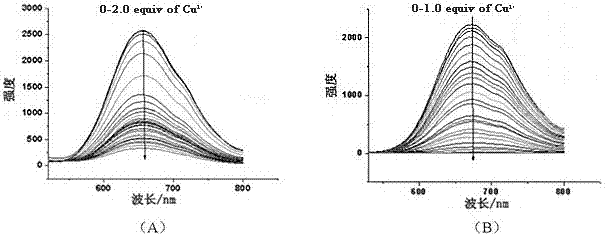
[0025] (1) At a concentration of 1.0×10 -5 mol / L complex (I) or complex (II) in acetonitrile solution, add a series of different amounts of Cu 2+ ions, and measured their ultraviolet-visible absorption spectra in the wavelength range of 200-800 nm, the results are shown in figure 1 . Such as figure 1 As shown in (A), the absorbance of complex (Ⅰ) increases with Cu at the wavelength of 240nm and 380nm 2+ The increase of ion concentration (0→3.0 times) increases; as figure 1 As shown in (B), the absorbance of complex (Ⅱ) increases with Cu at 250nm and 450nm 2+ The increase of ion concentration (0→1.0 times) increases, at 310nm wavelength with Cu 2+ decrease with increasing ion concentration.
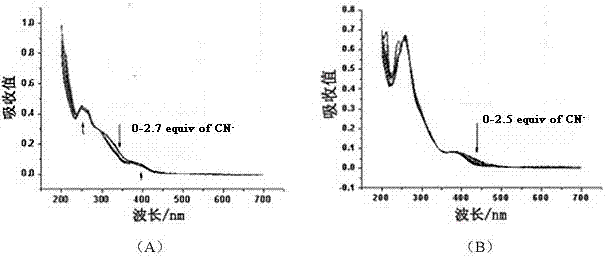
[0026] (2) At a concentration of 1.0×10 -5 A series of different amounts of CN - ions, and measured their ultraviolet-visible absorption spectra in the wavelength range of 200-800 nm, the results are shown in figure 2 . Such as figure 2 As shown in (A), the absorbance of com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com