Method for resource utilization of fluoroform
A technology of trifluoromethane and fluorodichloromethane, applied in chemical instruments and methods, chlorodifluoromethane production, organic chemistry, etc., can solve the problems of many by-products, serious carbon deposition, high reaction temperature, etc. Effects of cost, life extension, and product selectivity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
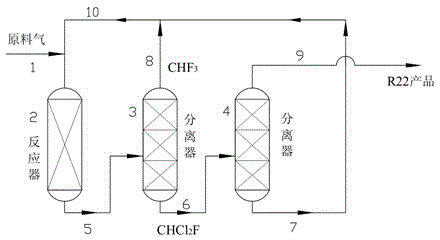
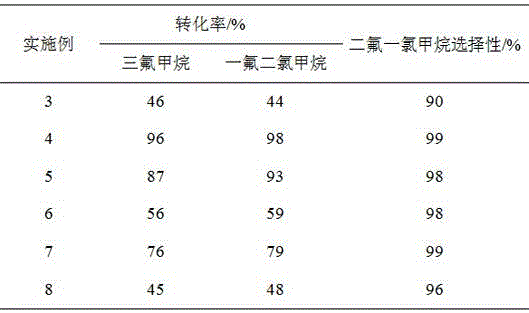
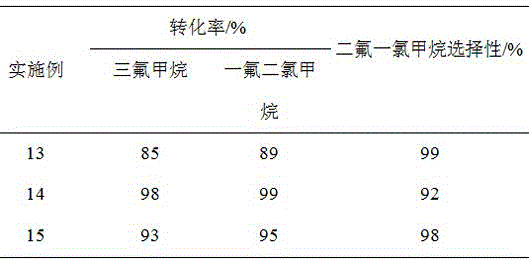
[0029] Pass trifluoromethane and fluorodichloromethane at a ratio of 1:1 (molar ratio) into a reactor containing 50ml of pretreated chromium trioxide catalyst, at a temperature of 350°C, a pressure of 2bar, and a space velocity of 2000h -1 react under the conditions. The pretreatment process of chromium trioxide is fluoridation treatment at 400°C for 2 hours in a mixed atmosphere of 10% hydrogen fluoride and 90% nitrogen, and finally 5 hours at 400°C with hydrogen fluoride. The conversion rate of trifluoromethane is 89%, and the selectivity of difluorochloromethane is 98%. In addition to some unreacted trifluoromethane and monofluorodichloromethane, there are traces of methane and CO in the tail gas. 2 Wait for gas. After the tail gas separates and collects difluorochloromethane, the remaining gas circulates into the reactor to continue to react with trifluoromethane.
Embodiment 2
[0031] Pass trifluoromethane and trichloromethane at a ratio of 2:1 (molar ratio) into a reactor containing 50ml of pretreated chromium trioxide catalyst, at a temperature of 350°C, a pressure of 2bar, and a space velocity of 2000h -1 react under the conditions. The pretreatment process of chromium trioxide is fluoridation treatment at 400°C for 2 hours in a mixed atmosphere of 10% hydrogen fluoride and 90% nitrogen, and finally 5 hours at 400°C with hydrogen fluoride. The conversion rate of trifluoromethane is 95%, and the selectivity of difluorochloromethane is 95%. In addition to some unreacted trifluoromethane and monofluorodichloromethane, there are traces of methane and CO in the tail gas. 2 Wait for gas. After the tail gas separates and collects difluorochloromethane, the remaining gas circulates into the reactor to continue to react with trifluoromethane.
Embodiment 3
[0032] Example 3 catalyst preparation
[0033] 10.09g Sm(NO 3 ) 3 Dissolve in 205ml of distilled water to make impregnating solution, impregnate on 100g of MgO carrier with high specific surface area, age for 12h after impregnating for 5 hours, dry the water in an oven at 110°C for 12h, and dry at 400°C N 2 Roasting under the atmosphere for 5h, the prepared 5.2wt%Sm 2 o3 / MgO catalyst. The prepared catalyst was evaluated according to the raw material composition, pretreatment process and reaction conditions of Example 1, and the evaluation results are shown in Table 1.
[0034] 14.04g La(NO 3 ) 3 6H 2 O was dissolved in 225ml of distilled water to make a solution, and 100g of high specific surface area Al 2 o 3 Dispersed in La(NO 3 ) 3 solution, a suspension was formed, to which 20% NH 3 water solution, and at the same time turn on the magnetic stirring until the pH = 9~11, stop the reaction, let it stand for aging for 12 hours, then carry out suction filtration, dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com