Mutant of schistosoma japonicum glutathione-S-transferase and application thereof
A technology of glutathione and transferase, applied in the field of enzyme genetic engineering, can solve the problems of not being able to block repeated infection of schistosomiasis, endangering human health, and generally low level of immune protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Construction and protein purification of sj26GST and its variant Q67E prokaryotic expression vector
[0028] Using the plasmid pALEX as a template, the coding sequence of wild-type GST was amplified by PCR. The upstream primer used is: 5'-catgcc atgggc atg tcc cct atactaggt tattgg-3' introduces the NcoI restriction site (underline), and the downstream primer is: 5'-ctgtagcggccgctctaga ctcgag ttt tgg agg atg gtc gcc ac-3', introduce the restriction site of XhoI (underlined). After purification, the PCR product was digested with NcoI / XhoI and inserted into the corresponding site of pET21d. Perform DNA sequencing on the recombinant expression vector to analyze the correctness of its reading frame and coding sequence. The sequence of the mutant protein Q67E is consistent with the N / C terminal sequence constructed by the wild-type GST, and only specific site-specific mutations are carried out. This constructed GST has an additional LEHHHHHH added at the C-ter...
Embodiment 2
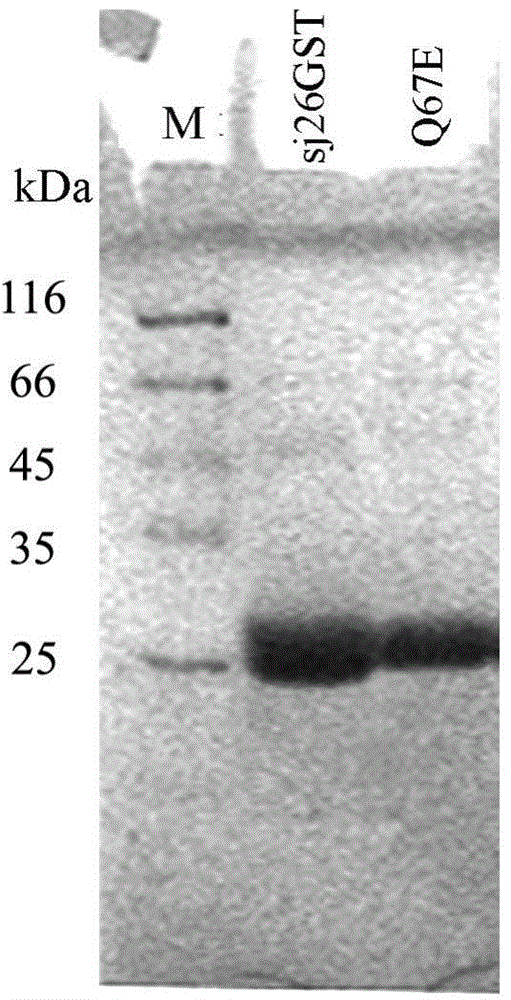
[0046]Example 2 Expression and purification of sj26GST and mutant enzyme Q67E of Schistosoma japonicum
[0047] The GST plasmid pET-21d-GST and its variant expression plasmid pET-21d-Q67E verified by sequencing were respectively transformed into Escherichia coli BL21 (DE3) host bacteria to induce expression. Pick positive transformants and shake culture overnight at 37°C and 200rpm in LB medium, then inoculate in LB medium (containing 100μg / ml ampicillin) and culture at 28°C and 250rpm until OD 600 is about 0.8, add IPTG with a final concentration of 0.2mM, and induce expression at 28°C for 6hr.
[0048] The fermentation broth was centrifuged at 6000rpm and 4°C for 10min, and the cells were resuspended in 20mL of buffer solution (50mM Na2HPO4-NaH2PO4, pH8.0, 150mM NaCl, 1mM PMSF), and the soluble protein was recovered by centrifugation after sonication.
[0049] The protein sample was loaded on a nickel metal chelate affinity chromatography column (1.6cm×5cm) equilibrated wit...
Embodiment 3
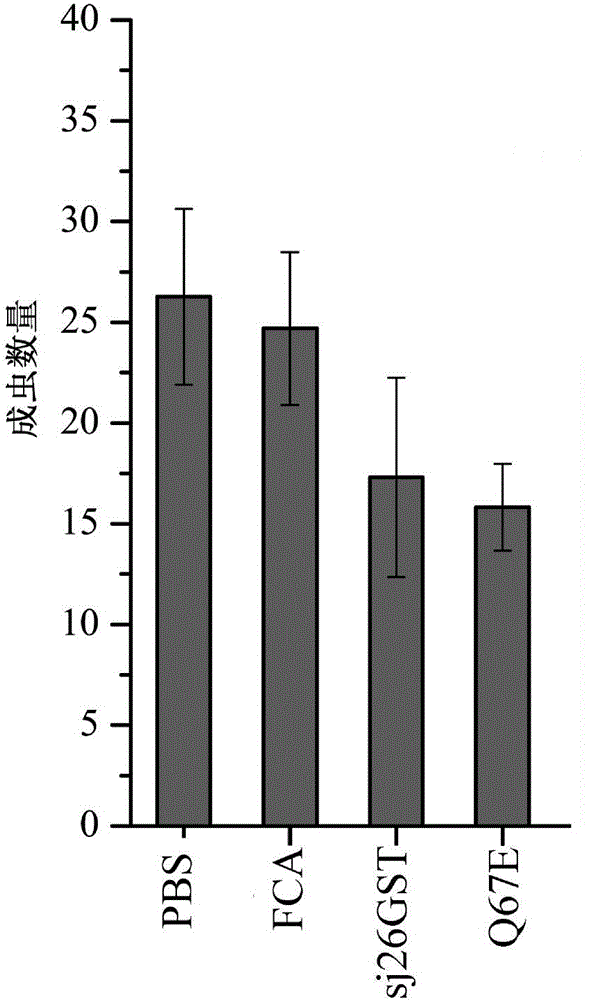
[0050] Example 3 Detection of Enzyme Activity of Schistosoma japonicum sj26GST and its variants
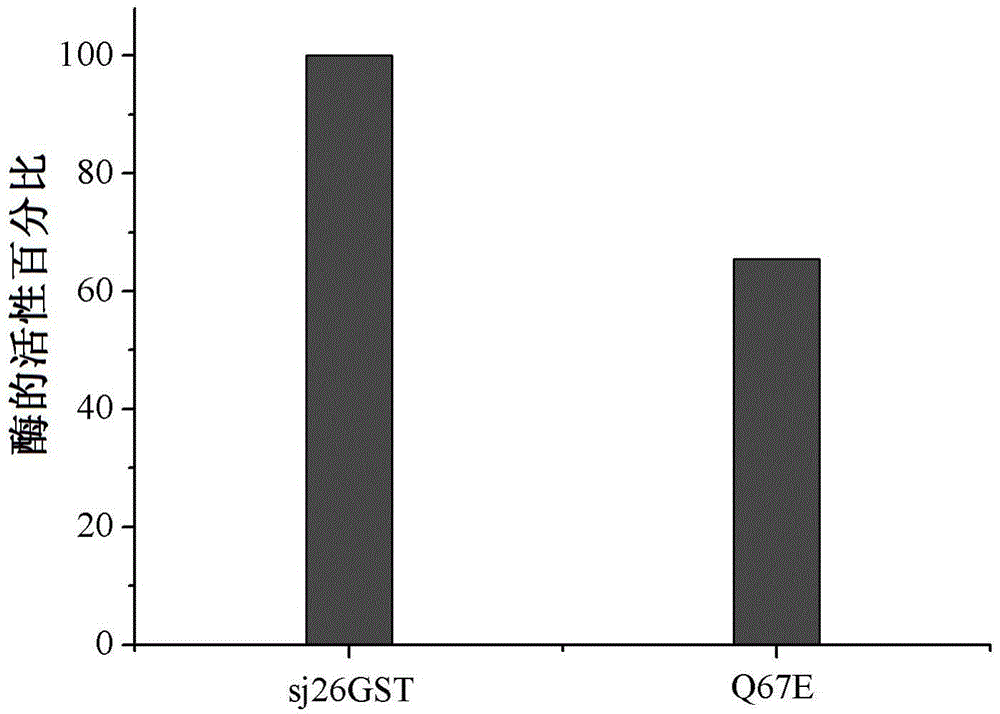
[0051] Add the enzyme solution to the 0.1M potassium phosphate solution at pH 6.5 containing 1mM 2,4-dinitrochlorobenzene (CDNB) and 1mM reduced glutathione (GSH), measure the change of its optical analysis value at 340nm, and repeat the experiment Three times (Habig, W.H. and Jakoby, W.B. Assays for differentiation of glutathione S-transferases. Meth. Enzymol. 1981, 77:398-405). Taking the highest activity of the wild-type sj26GST as 100%, the ratio of the activity of the mutant enzyme to the activity of the wild-type enzyme was calculated. ( figure 2 )
[0052] Determination of the apparent Michaelis constant of GST to GSH and CDNB: the reaction system was fixed at 1.0ml, the final concentration of CDNB in the system was 1.0mmol / L, and the final concentration range of GSH was 40, 50, 66, 100, 200μmol / L; The final concentration of GSH in the system was 1.0mmol / L, and the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com