Scutellaria barbata extract as well as preparation method and application thereof
A technology for extracts and medicines of Scutellaria barbata, which is applied in the directions of pharmaceutical formulations, plant raw materials, medical preparations containing active ingredients, etc. Replication activity and other issues, to achieve a good inhibitory effect, the effect of great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The method for preparing compound 1-4 from barbata (Scutellaria barbata):
[0031] Scutellaria barbata (Scutellaria barbata) whole plant (3.5Kg), dried in the sun, pulverized, leached 3 times with 95% ethanol at room temperature, each time for 1 day, combined the concentrated solutions to obtain crude extract (362g), respectively washed with petroleum ether , ethyl acetate, n-butanol extraction, ethyl acetate layer (183g) directly through macroporous resin (D101), with ethanol / water (0:100, 10:90, 20:80, 30:70, 40:60 , 50:50, 60:40, 70:30, 80:20:, 95:5, 100:0, V / V) gradient elution, one part was collected for each 450mL, and the same part was combined for TLC detection to obtain a total of six Fraction Fr.1-6. Parts Fr.3-5 were subjected to silica gel column chromatography (200-300 mesh), Sephadex LH-20, C 18 Column and semi-preparative liquid chromatography column, repeated separation, the following 4 compounds were obtained.
[0032] Compound 1 (new compound):
[...
Embodiment 2
[0049] Embodiment 2 Pharmacodynamic related experiments of the 4 compounds obtained in Example 1
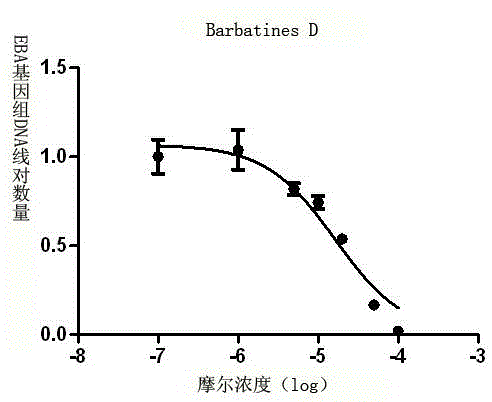
[0050] Determination of inhibitory activity of compounds 1-4 of the present invention on EBV cleavage and replication.
[0051] (1) Cell culture: in vitro culture of P3HR-1 cells (primary exudative lymphoma cell line, containing EBV in latent infection stage), using 10% fetal bovine serum, streptomycin (100 μg / ml), The RPMI1640 medium of penicillin (100 units / mL) was maintained at 37° C. under the condition of 5% carbon dioxide concentration and subcultured.
[0052] (2) Drug intervention: adjust the density of P3HR-1 cells in the logarithmic growth phase to 3×10 5 cells / mL, use 20ng / mL of 12-O-tetradecanoylphorbol-13-ethyl ester (TPA) and sodium butyrate (0.3mM) to induce P3HR-1 cells to enter the lytic replication phase. DMSO was used to prepare drug solutions with different concentrations (150, 100, 50, 20, 10, 1, 0.1, 0 μM) of the compounds 1-4 to be tested. After P3HR-1 c...
Embodiment 3
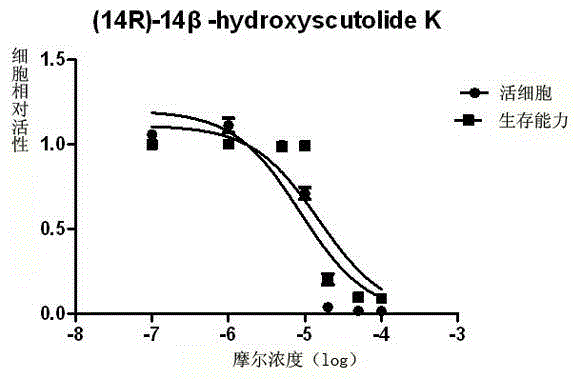
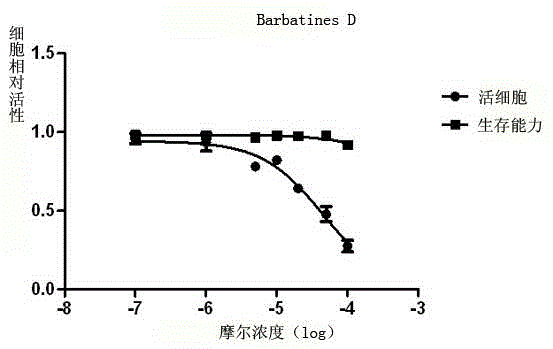
[0059] Example 3 Compound 1-4 of the present invention is tested for host cell toxicity
[0060] (1) Cell culture: P3HR-1 cells (primary exudative lymphoma cell line, containing EBV in latent infection stage) were cultured in vitro. The RPMI1640 medium containing 10% fetal bovine serum, 400ug / ml G418, and 100ng / ml doxycycline was used for routine maintenance and passage at 37°C and 5% carbon dioxide concentration.
[0061] (2) Test method: adjust the density of P3HR-1 cells in the logarithmic growth phase to 3×10 5 cells / ml, the cells were treated with different concentrations of compound 1-4 (150, 100, 50, 20, 10, 1, 0.1, 0 μM), and three parallel wells were set up for each concentration. After 2 days, trypan blue staining was used, and light Count the number of viable cells under a microscope.
[0062] (3) Result processing: according to the formula: relative toxicity = 1-livecell compound+ / live cell compound_ Calculate the relative toxicity and median lethal dose (CC) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com