Application of swine enzootic hyopneumoniae vaccine strain
A technology of mycoplasma pneumoniae and mycoplasma hyopneumoniae, which is applied in the direction of bacterial antigen components and antibacterial drugs, can solve the problems of difficult popularization and expensive vaccines, and achieve the effect of simple vaccine production method, safe and effective vaccine, and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation of swine mycoplasma pneumonia inactivated vaccine (CJ strain):
[0021] 1 strain:
[0022] 1.1 The bacterial classification used to manufacture this product is Mycoplasma hyopneumoniae CJ strain (the said Mycoplasma hyopneumoniae vaccine strain, its classification is called Mycoplasma hyopneumoniae (Mycoplasma hyopneumoniae), and the bacterial strain number is CJ, has been deposited in China Microorganism Strain Preservation Management Committee General Microbiology Center, the preservation number is CGMCC NO: 9909, and the preservation date is November 06, 2014). The strain used for inspection is Mycoplasma suis pneumonia lung tissue virus, which was isolated, identified, and tested by Xinjiang Tiankang Animal Husbandry Biotechnology Co., Ltd. custody and supply;
[0023] 1.2 Bacteria for production:
[0024] 1.2.1 Morphology and biochemical characteristics: smeared with liquid culture, stained by Gimu Sax, under high magnification, typi...
Embodiment 2
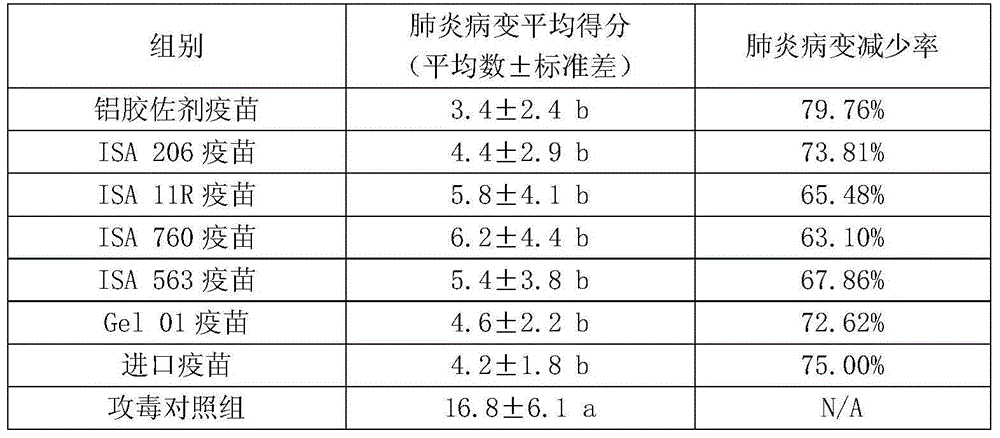
[0053] Embodiment 2: Observation of immune effect of swine mycoplasma pneumonia inactivated vaccine (CJ strain)
[0054] Vaccine: Mycoplasma pneumonia inactivated vaccine (CJ strain) (aluminum hydroxide adjuvant), Mycoplasma pneumonia inactivated vaccine (CJ strain) (ISA 206), Mycoplasma pneumonia inactivated vaccine (CJ strain) (ISA 11R) , Mycoplasma pneumonia inactivated vaccine (CJ strain) (ISA 760), Mycoplasma pneumonia inactivated vaccine (CJ strain) (ISA 563), Mycoplasma pneumonia inactivation vaccine (CJ strain) (Gel 01), imported Mycoplasma suis Pneumonia inactivated vaccine;
[0055] Bacteria used for inspection: Mycoplasma hyopneumoniae lung tissue virus, isolated, identified, kept and supplied by Xinjiang Tiankang Animal Husbandry Biotechnology Co., Ltd.;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com