Copper-containing complex catalyst and preparation method and application thereof
A technology of copper complexes and catalysts, which is applied in the field of catalysts, can solve problems such as high requirements for reaction equipment and difficult wastewater treatment, and achieve the effects of simple preparation methods, long processes, and good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
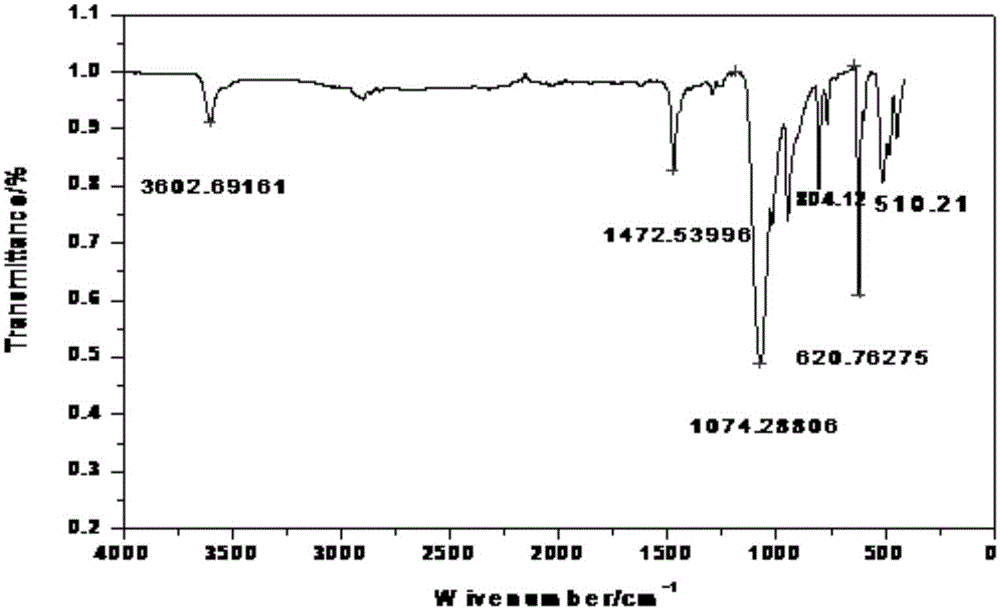
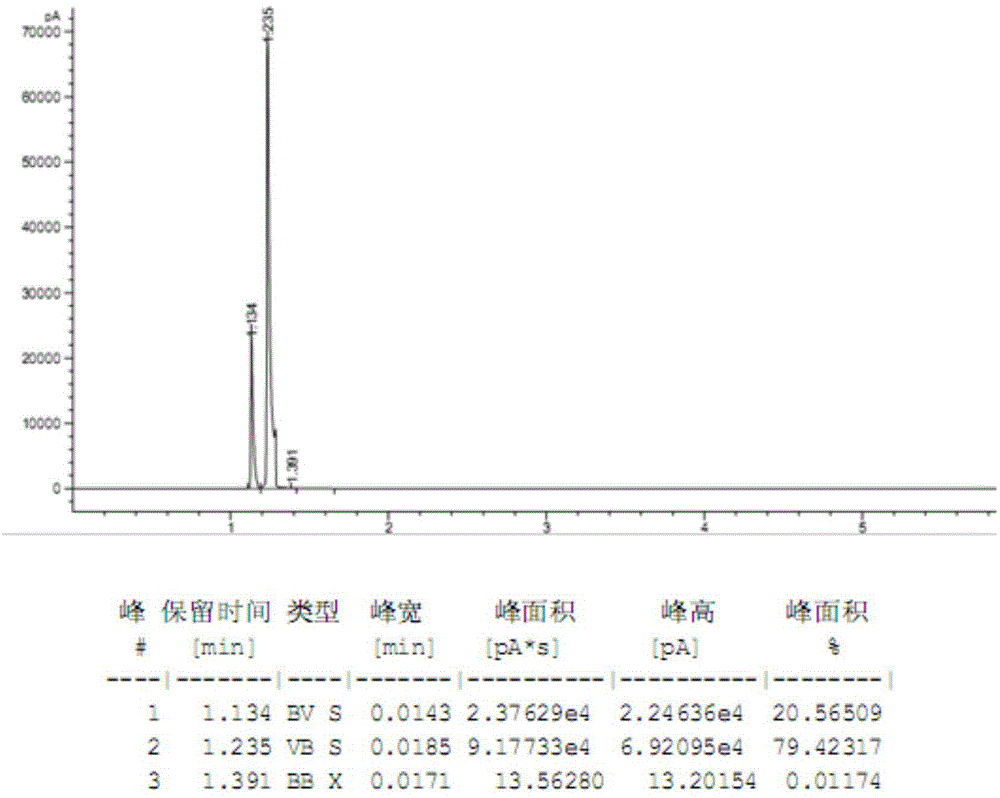
Image
Examples
Embodiment 1
[0024] First take copper nitrate in a 250mL large beaker, add 80mL of water, then take sodium carbonate, (so that the ratio of copper nitrate and sodium carbonate is 1:1) in a 100mL small beaker, add 80mL of water, stir to fully dissolve the two, and assemble the titration device. While stirring the copper nitrate solution with a magnet, slowly add sodium carbonate dropwise to it with a constant pressure dropping funnel, a green precipitate appears in the blue copper nitrate solution, and with the continuous addition of sodium carbonate, the precipitate More and more, the overall solution turned into a green mixture. After the sodium carbonate titration is completed, continue to stir for 30 minutes to fully react the sodium carbonate and copper nitrate. After stirring, the mixed solution was randomly separated into a precipitate layer and an aqueous layer. Assemble a suction filtration device, suction filter the obtained mixed solution to obtain a green solid, put the filter...
Embodiment 2
[0029] First take copper acetate in a 250mL large beaker, add 80mL of water, then take sodium carbonate, (the ratio of the amount of copper acetate and sodium carbonate taken is 1:2) in a 100mL small beaker , add 80mL of water, stir to fully dissolve both, and assemble the titration device. While stirring the copper acetate solution with a magnet, slowly add sodium carbonate dropwise to it with a constant pressure dropping funnel, a green precipitate appears in the copper chloride solution, and with the continuous addition of sodium carbonate, the precipitation becomes more and more More and more, the overall solution turns into a green mixture. After the sodium carbonate titration is completed, continue to stir for 30 minutes to fully react the sodium carbonate and copper chloride. After stirring, the mixed solution was randomly separated into a precipitate layer and an aqueous layer. Assemble a suction filtration device, and suction-filter the obtained mixed solution to ob...
Embodiment 3
[0034] Take copper chloride in a 250mL large beaker, add 80mL of water, then take sodium carbonate, (the ratio of the amount of copper acetate and sodium carbonate taken is 1:1) in a 100mL small beaker , add 80mL of water, stir to fully dissolve both, and assemble the titration device. While stirring the copper chloride solution with a magnet, slowly add sodium carbonate dropwise to it with a constant pressure dropping funnel, a green precipitate appears in the copper solution, and with the continuous addition of sodium carbonate, the precipitate becomes more and more More, the overall solution turns into a green mixture. After the sodium carbonate titration is completed, continue to stir for 30 minutes to fully react the sodium carbonate and copper acetate. After stirring, the mixed solution was randomly separated into a precipitate layer and an aqueous layer. Assemble a suction filtration device, and suction-filter the obtained mixed solution to obtain a green solid, and p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com