Preparation method of alpha-Fe2O3 nanoparticles
A nanoparticle and anion technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of difficult industrial production, difficult control of conditions, serious environmental pollution, etc., to increase ionic strength, The effect of low cost and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
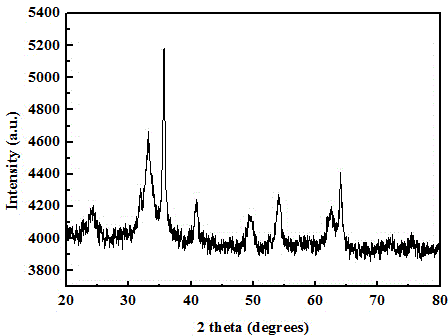
Image
Examples
preparation example Construction
[0020] In the preparation method of the present invention, the mixing mode of the inorganic alkali dispersion solution and the iron dispersion solution determines the acid-base environment, particle environment and reaction intensity during the reaction. Since the reaction environment and reaction intensity will affect the local reaction system, the shape and particle size of the reaction product are determined. In the present invention, the inorganic alkali dispersion solution must be added dropwise to the iron source dispersion solution. If the addition is reversed, only amorphous iron oxide particles can be generated, and the particle size is relatively large. The concentration of iron particles in the iron source dispersion solution and the concentration of hydroxide ions in the inorganic alkali dispersion solution will affect the utilization rate and reaction intensity of the particles in the reaction. Generally, the concentration of iron particles is in the range of 0.01~...
Embodiment 1
[0023] 1. Add 4.8g ferric ammonium sulfate dodecahydrate with 75mL concentration to 1.5mol L -1 Na 2 SO 4 The solution is dispersed and dissolved, and stirred evenly to obtain NH 4 Fe(SO 4 ) 2 Dispersion solution, the concentration of iron ions in the dispersion solution is 0.133mol / L.
[0024] 2. Add 1.2g NaOH to 75 mL to a concentration of 1.5mol L -1 Na 2 SO 4 The solution is dispersed and dissolved, and stirred evenly to obtain a NaOH dispersion solution, and the concentration of sodium hydroxide in the dispersion solution is 0.399mol / L.
[0025] 3. Add NH 4 Fe(SO 4 ) 2 The dispersion solution was transferred to a three-necked flask, placed in a reflux device in a glycerin bath and heated to 60°C, and the NaOH dispersion solution was added to the NH 4 Fe(SO 4 ) 2 In the dispersion solution, a brown precipitate is formed, continue to stir and reflux for 5 hours, centrifuge, wash the precipitate with water, water-ethanol mixture, and absolute ethanol, and vacuum...
Embodiment 2
[0027] 1. Add 2.7g ferric chloride hexahydrate with 75mL concentration to 1.0mol L -1Disperse and dissolve the sodium chloride solution, and stir evenly to obtain a ferric chloride dispersion solution. The concentration of iron ions in the dispersion liquid is 0.133mol / L.
[0028] 2. Use 75 mL of 1.2 g of sodium hydroxide to a concentration of 1.0 mol L -1 Disperse and dissolve the sodium chloride solution, stir to obtain a sodium hydroxide dispersion solution, and the concentration of sodium hydroxide in the dispersion liquid is 0.399mol / L.
[0029] 3. Ferric chloride dispersion solution is transferred in the three-necked flask, is placed in the reflux device of glycerine bath and is heated to 80 ℃, under constantly stirring, sodium hydroxide dispersion solution is added in the middle of ferric chloride dispersion solution, has Brown precipitate is formed, continue to fully stir and reflux for 5 hours, centrifuge, wash the precipitate with water, water-ethanol mixture, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystal size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com