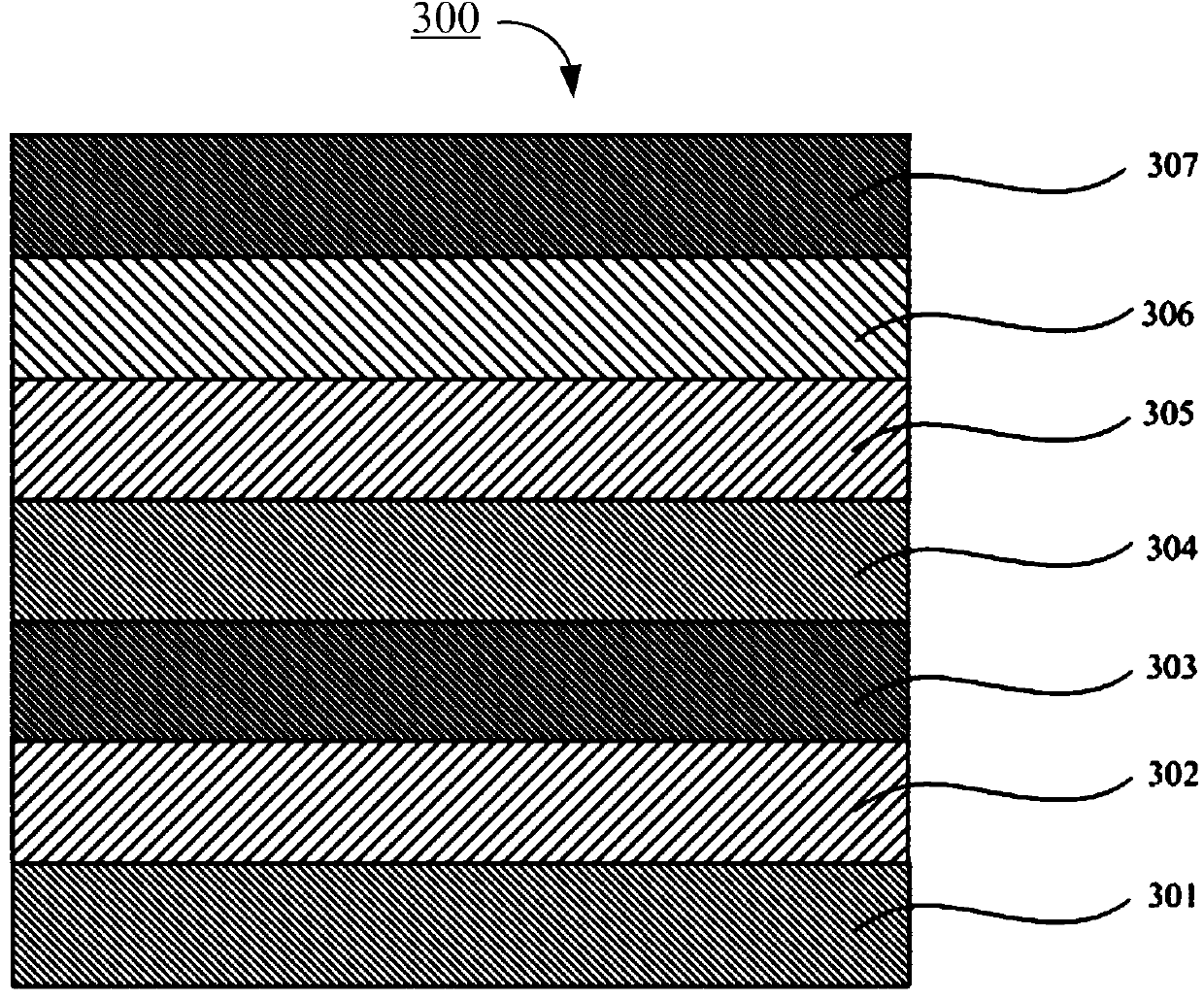
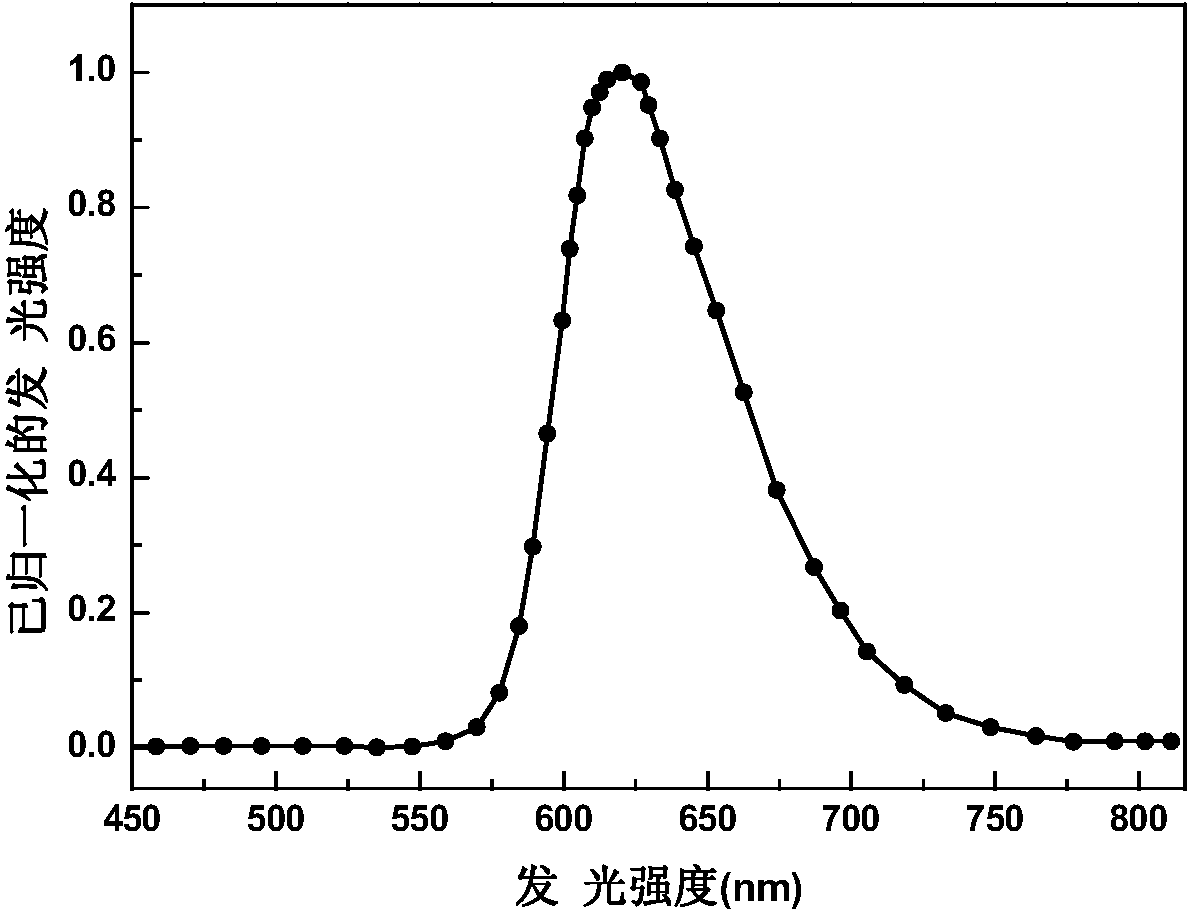
Red phosphorescent iridium complex and preparation method thereof, and organic electroluminescence device
A technology of iridium metal complexes and red phosphorescence, applied in the fields of electric solid-state devices, organic chemistry, luminescent materials, etc., can solve the problems of color purity and backwardness that are rarely achieved in deep red and deep green light
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
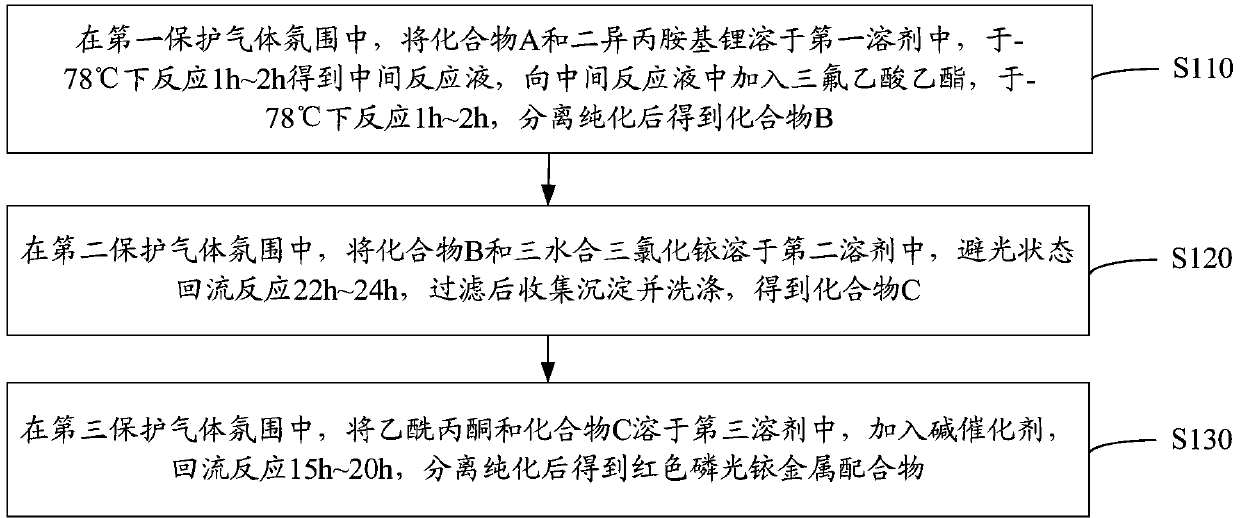
[0042] see figure 1 , a preparation method of a red phosphorescent iridium metal complex, comprising the following steps.
[0043] S110: In the first protective gas atmosphere, dissolve compound A and lithium diisopropylamide in the first solvent, react at -78°C for 1h to 2h to obtain an intermediate reaction liquid, and add ethyl trifluoroacetate to the intermediate reaction liquid The ester was reacted at -78°C for 1h to 2h, and compound B was obtained after separation and purification.
[0044] Compound A and lithium diisopropylamide ( ) in a molar ratio of 1:1.1 to 1.3, and the molar ratio of ethyl trifluoroacetate to lithium diisopropylamide is 1:1.
[0045] The structural formula of compound A is: The structural formula of compound B is: R is hydrogen or methyl. When R is a methyl group, R can replace the hydrogen on the 3-, 4-, 5-, 6-, 7- or 8-position of the isoquinoline ring. For example, compound B can specifically be 1-(4'-trifluoroacetylphenyl) isoquinolin...
Embodiment 1
[0083] Red Phosphorescent Iridium Metal Complex Bis[1-(4'-trifluoroacetylphenyl)isoquinoline-N,C 2 '] (acetylacetonate) iridium synthesis.
[0084] (1) Synthesis of ligand 1-(4'-trifluoroacetylphenyl)isoquinoline.
[0085]
[0086] At -78°C, in a nitrogen atmosphere, 5 mL of a tetrahydrofuran solution containing 2.84 g (10 mmol) of 1-(4'-bromophenyl) isoquinoline was added to 1.29 g (12 mmol) of lithium diisopropylamide and 10 mL of In the mixed solution composed of tetrahydrofuran, stir and react for 1 h to obtain an intermediate reaction solution. A mixture consisting of 1.43 mL (12 mmol) of ethyl trifluoroacetate and 5 mL of tetrahydrofuran was added dropwise to the intermediate reaction solution, and the reaction system was maintained at -78° C., and stirred for 1 h. After the reaction was completed, the reaction system was naturally raised to 0°C, stirred and reacted for 0.5 h, then an appropriate amount of saturated ammonium chloride aqueous solution was added, and ...
Embodiment 2
[0108] Red Phosphorescent Iridium Metal Complex Bis[1-(4'-trifluoroacetylphenyl)-3-methylisoquinoline-N,C 2 '] (acetylacetonate) iridium synthesis.
[0109] (1) Synthesis of ligand 1-(4'-trifluoroacetylphenyl)-3-methylisoquinoline
[0110]
[0111] At -78°C, under a nitrogen atmosphere, 5 mL of a tetrahydrofuran solution containing 2.98 g (10 mmol) of 1-(4'-bromophenyl) 3-methylisoquinoline was added to 1.29 g (12 mmol) of diisopropylamine Lithium base and 10mL of tetrahydrofuran in a mixed solution, stirred for 1 h to obtain an intermediate reaction solution. A mixture consisting of 1.43 mL (12 mmol) of ethyl trifluoroacetate and 5 mL of tetrahydrofuran was added dropwise to the intermediate reaction solution, and the reaction system was maintained at -78° C., and stirred for 1 h. After the reaction system naturally rose to 0°C, stirred and reacted for 0.5 h, an appropriate amount of saturated ammonium chloride aqueous solution was added, extracted with ethyl acetate, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Maximum lumen efficiency | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com