Method for detecting living bacteria body of listeria monocytogenes in sample
A technology for Listeria monocytogenes and sample detection, which is applied in the field of biomedicine and can solve the problem of inability to distinguish between live and dead bacteria of Listeria monocytogenes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0016] Wherein, as a particularly preferred embodiment of the present invention, the antibody capable of specifically binding to live Listeria monocytogenes is the California Bioscience monoclonal antibody numbered MC0007, which can be purchased from California Bioscience Available commercially under item number MC0007.
[0017] Wherein, the amount of the antibody may be 0.05-2 mg relative to each mg of magnetic balls.
[0018] Wherein, relative to the sample to be tested in liquid form per milliliter, the amount of the immunomagnetic balls may be 10-25 μg in terms of the weight of the magnetic balls.
[0019] Wherein, in step (3), the liquid used for elution may be a phosphate buffer containing 0.144-0.162 mol / L disodium hydrogen phosphate and 0.038-0.056 mol / L sodium dihydrogen phosphate.
[0020] Wherein, as a particularly preferred embodiment of the present invention, for the live cells of Listeria monocytogenes, the primers used for PCR amplification include the forward ...
preparation Embodiment 1
[0025] In Example 1 of this preparation, Antibody 1 was used to prepare immunomagnetic beads used in the method of the present invention.
[0026] Surface-modified superparamagnetic Fe with carboxyl groups activated by 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and N-hydroxysuccinimide (NHS) 3 o 4 Polystyrene composite microspheres (purchased from Shanghai Aorun Micro-Nano New Material Technology Co., Ltd., PM3-020 polymer magnetic balls, with a concentration of 10 mg / mL, a particle size of 180 nm, and carboxyl functional groups on the surface) were used as magnetic balls , take 2 mg of magnetic spheres and disperse them in the phosphate buffer solution, the concentration of the magnetic spheres in the dispersion is 2 mg / mL. Mix 0.2 mg of specific antibody (dissolved in 1 mL of phosphate buffer) with 2 mg of magnetic balls, keep in a shaker (200 r / min) for 3 hours at room temperature, and then wash by magnetic separation to remove the uncoupled magnetic balls. Surf...
Embodiment 1
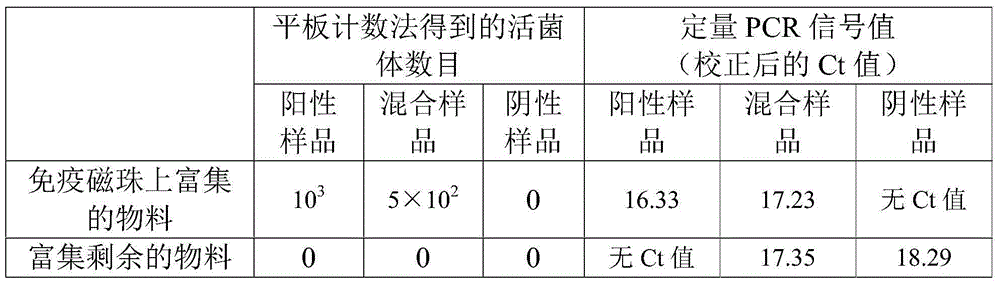
[0029] Cultivate Listeria monocytogenes (purchased from ATCC, product number 35152) in LB medium (tryptone 10g / L, yeast extract 5g / L, sodium chloride 10g / L) to logarithm period, adjust the cell concentration to 10 with LB medium 3 pc / L, as a positive sample containing viable Listeria monocytogenes.
[0030] The above positive samples were inactivated by high pressure (121°C, 15min) to obtain viable cells that did not contain Listeria monocytogenes but contained 10 3 Negative samples of / L dead bacteria.
[0031] Mix the positive sample and the negative sample in equal volumes to obtain 5×10 2 each / L of dead bacteria and 5×10 2 A mixed sample of viable cells / L.
[0032] Mix 1mL of positive samples, negative samples and mixed samples with immunomagnetic balls 1 (15 mg based on the weight of magnetic balls) to obtain the mixed materials, incubate at 29°C for 30 min, and then fix the immunomagnetic beads in a sorting magnetic field. Magnetic spheres, to elute the substances t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com