Application of triterpenoid compound in preparation of antitumor medicines
An anti-tumor drug, triterpenoid technology, applied in the field of biomedicine, can solve the problems of drug resistance, unsatisfactory drug effect, lack of selectivity, etc., and achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Prepares Tormentic acid from Raspberry palmatum
[0033] (1) 5Kg of the palm leaf raspberry 5Kg purchased will be pulverized and sieved, mixed in batches with 10 times the amount (mass) of ethanol with a volume fraction of 95% to extract three times under reflux, combine the extracts, filter, and use a rotating The evaporator concentrated and evaporated to dryness under reduced pressure at 40°C, recovered ethanol, and obtained the ethanol crude extract of raspberry palmate;
[0034] (2) the raspberry palmate ethanol crude extract extract prepared by step (1) is dissolved with 1.5L of methanol, then add an equal volume of sherwood oil solvent (i.e. add the volume of sherwood oil volume=crude extract+methanol ), fully vibrate and mix, and let stand for 2h. After the two-phase solvents are completely separated, suck out the petroleum ether phase in the upper layer; repeat the extraction three times, combine the petroleum ether extracts, concentrate under reduced ...
Embodiment 2
[0036] Example 2 Determine the structure of compound 1
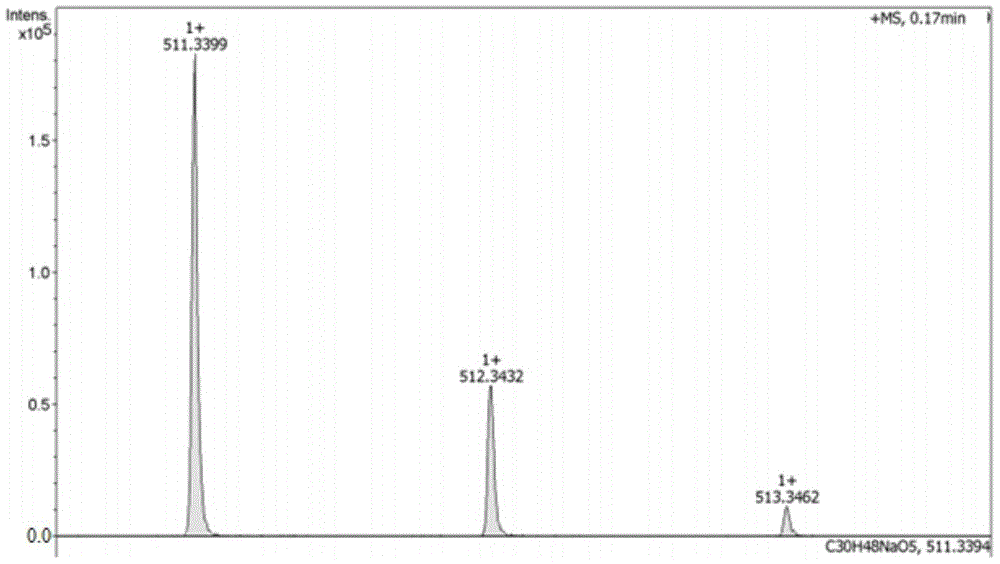
[0037] Compound 1 (trace amount) prepared in Example 1 was dissolved in chromatographic methanol, and analyzed by ESI-MS of Esquire HCTplus type large-capacity ion trap LC / MS. The mass-to-charge ratio m / z scanning range: 200 to 2000, obtained Sample positive ion mass spectrum ( figure 1 ). Dissolve compound 1 with pyridine, a deuterated reagent, and place it in a nuclear magnetic resonance tube. Using a Bruker DRX-400 nuclear magnetic resonance instrument, tetramethylsilane (TMS) is used as an internal standard to measure its hydrogen spectrum (1H-NMR), full decoupling Carbon spectrum (13C-NMR).
[0038] Compound 1 is a white amorphous powder. ESI-MS m / z:511[M+Na] + (Introduction molecular formula is C30-H48-O5). 1 H-NMR (C 5 D. 5 N)δ: 1.02(3H, s), 1.11(3H, s), 1.13(3H, s), 1.15(3H, d, J=6.5Hz), 1.30(3H, s), 1.44(3H, s) ,1.74(3H,s),3.08(1H,s,18-H),3.41(1H,d,J=9.3Hz,3-H),4.13(1H,m,2-H),5.59(1H, brs,12-H). 13 C-N...
Embodiment 3
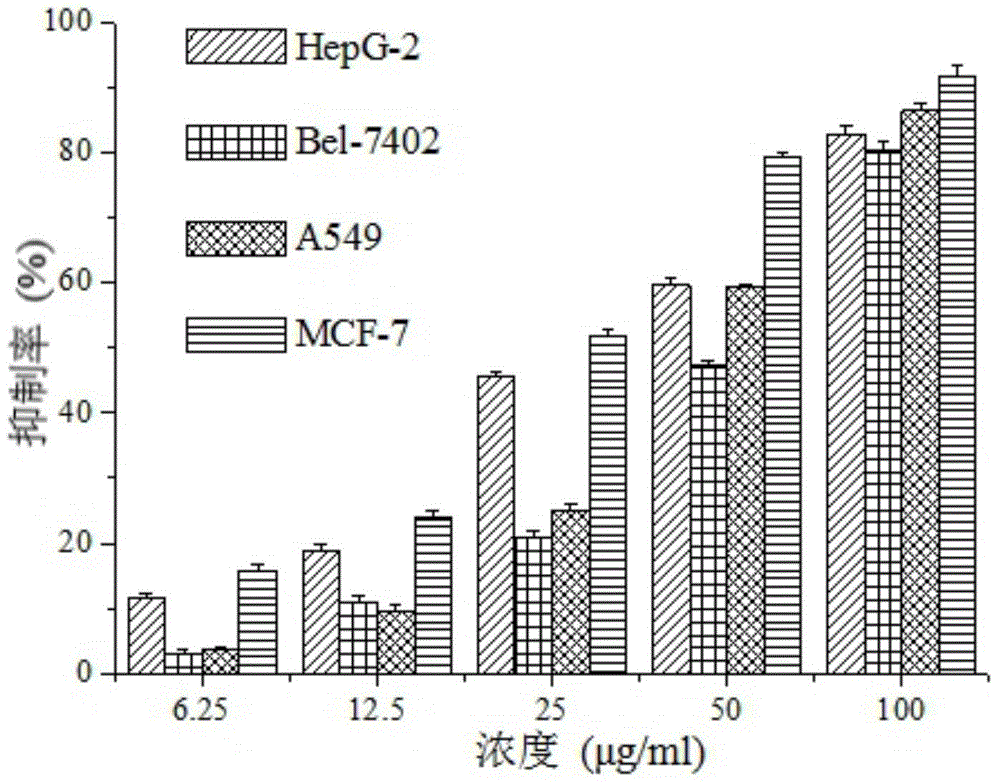
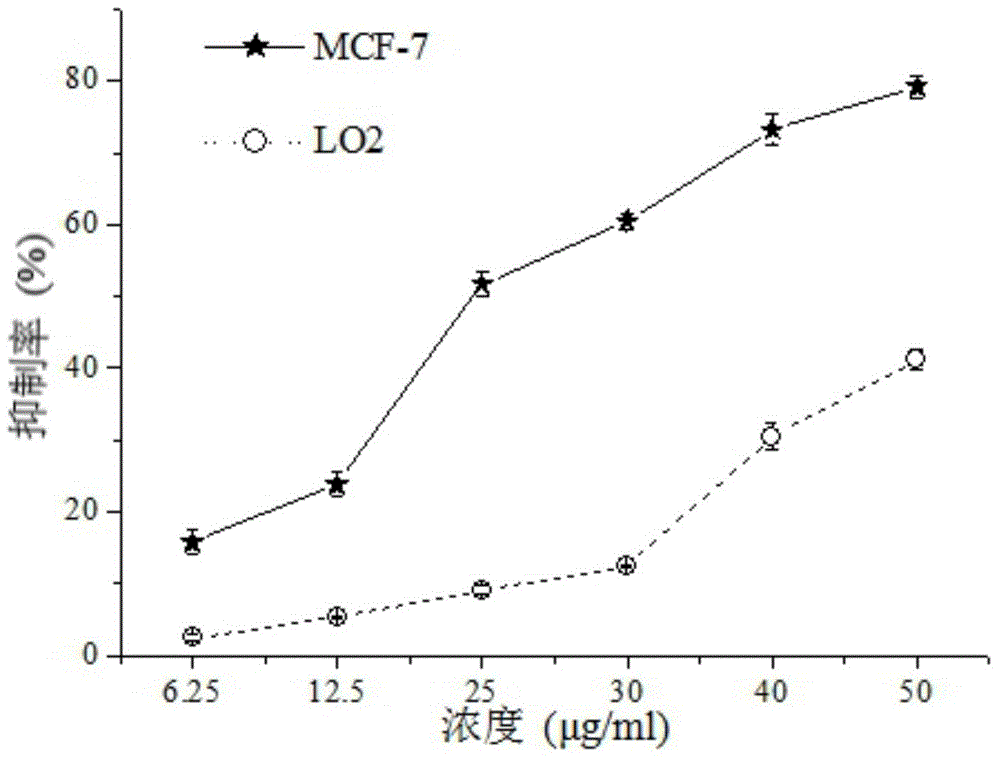
[0039] Example 3 Tormentic acid inhibits the proliferation of tumor cells of different histological types
[0040] Vigorously growing human liver cancer HepG-2 cells, human liver cancer Bel-7402 cells, human lung cancer A549 cells and human breast cancer MCF-7 cells were taken respectively, centrifuged at 1000rpm for 5min, discarded the supernatant, and used fetal tissue containing 10% mass fraction. The DMEM medium of bovine serum adjusted the cell number to 3×10 4 / mL, 100 μL was inoculated in a 96-well culture plate, placed in 5% CO 2 After culturing at 37°C for 24 h in the incubator, the supernatant was discarded, and DMEM medium containing Tormentic acid was added to each group (the final concentrations of Tormentic acid were 6.25 μg / mL, 12.5 μg / mL, 25 μg / mL, 50 μg / mL, respectively). mL and 100 μg / mL). At 37°C, 5% CO 2 Culture in an incubator for 24 hours, discard the supernatant, wash twice with PBS, add 200 μL of serum-free DMEM medium and 20 μL of thiazolyl blue (MT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com