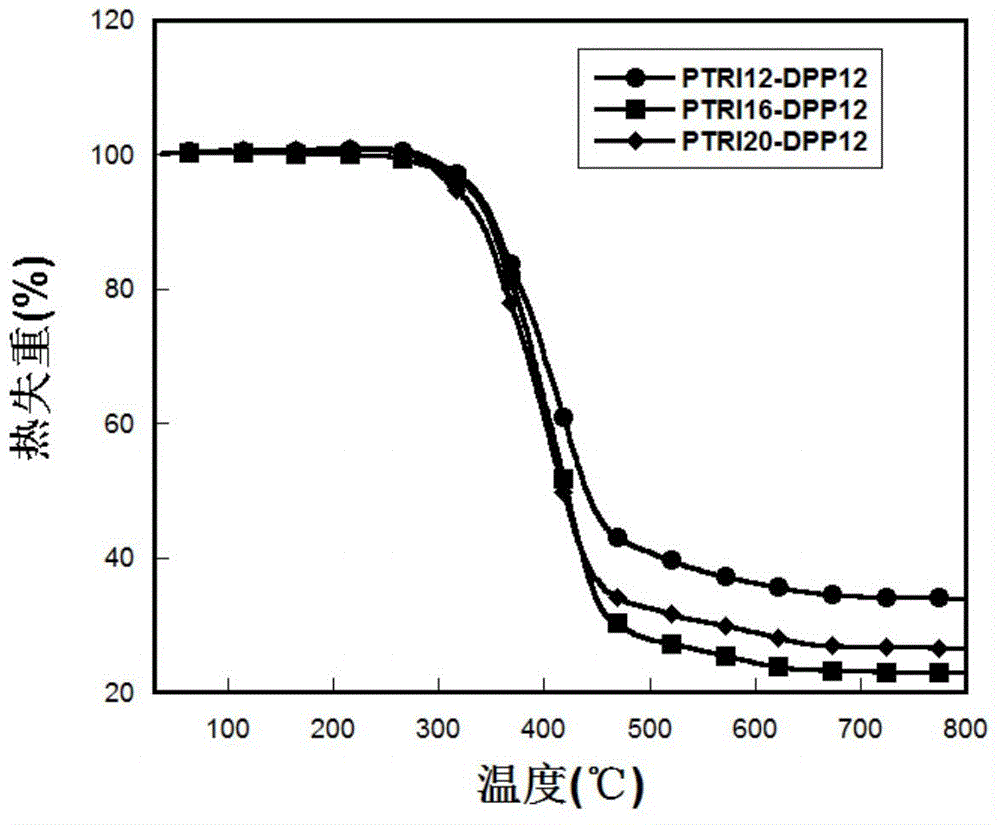
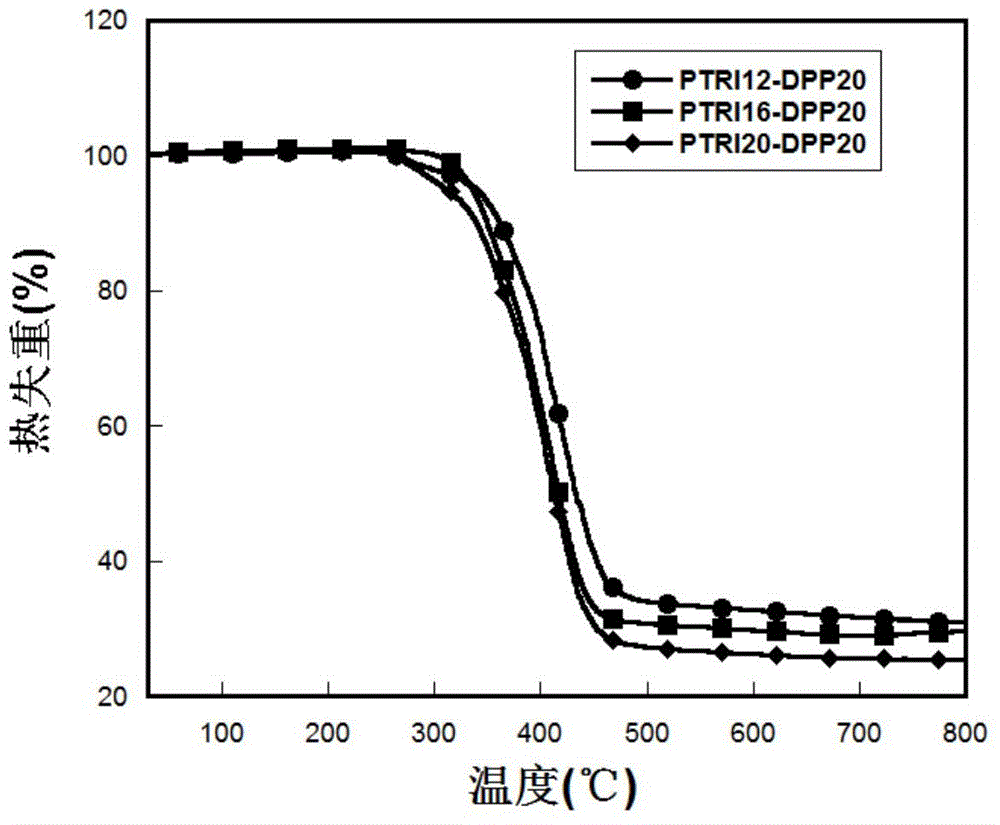
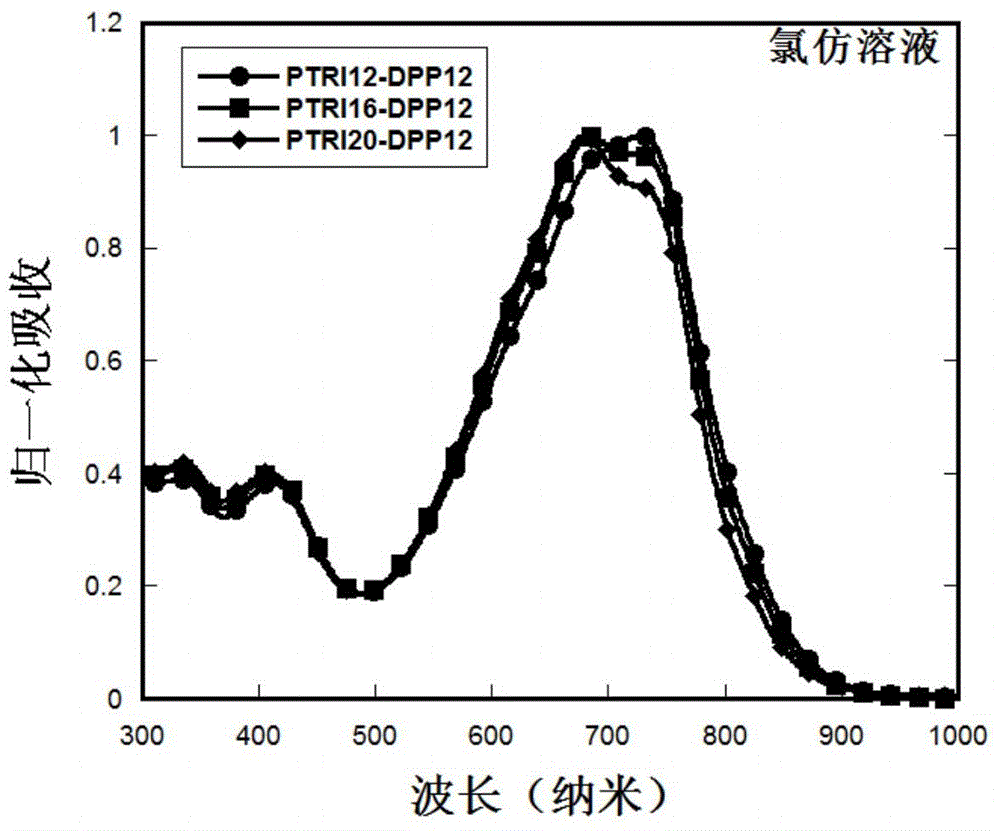
Organic semiconductor material and preparation method and application thereof
An organic semiconductor and conjugation technology, applied in the field of organic semiconductor materials and their preparation, can solve problems such as low efficiency of organic solar cells, and achieve the effects of being beneficial to charge transport, good planarity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]Example 1, 4,4'-(2,6-dibromobenzo[1,2-B:4,5-B']dithiophene-4,8-diyl)bis(1-2-butyl Synthesis of octyl)-1H-1,2,3-triazole) (abbreviated as DBrTRI12)
[0043] The chemical reaction process is shown in the following formula, and the specific reaction steps and reaction conditions are as follows:
[0044] Among them, (I) oxalyl chloride, dichloromethane, room temperature for 12 hours; (II) dimethylamine hydrochloride, triethyl, dichloromethane, room temperature for 12 hours; (Ⅲ) NBS, DMF, room temperature for 6 hours; (IV )-78°C, tetrahydrofuran, n-butyllithium, room temperature for 12 hours; (Ⅴ)-78°C, trimethylsilylacetylene, tetrahydrofuran, return to room temperature for 1 hour, then add anhydrous stannous chloride, 50°C, 12 hours; (VI) azidoalkyl chain, anhydrous potassium carbonate, cuprous iodide, tetrahydrofuran, water, acetonitrile, reflux.
[0045] (1) Dissolve 2,5-dibromothiophene-3-carboxylic acid dimethylamide (compound 3, 15.6g, 50mmol) synthesized according ...
Embodiment 2
[0051] Example 2, 4,4'-(2,6-dibromobenzo[1,2-B:4,5-B']dithiophene-4,8-diyl)bis(1-2-hexyldecane Synthesis of -1H-1,2,3-triazole) (abbreviated as DBrTRI16)
[0052] Synthetic method is consistent with embodiment 1. A light yellow powder (DBrTRI16, 1.7 g, 92%) was obtained.
[0053] The NMR data of the product are as follows: 1 H NMR (600MHz, CDCl 3 ),δ(ppm):8.05(s,2H).7.96(s,2H),4.44(d,4H),2.07(m,2H),1.36-1.25(m,48H),0.89-0.85(m, 12H). 13 C NMR (150MHz, CDCl 3 ), δ(ppm): 144.23, 138.49, 135.05, 126.10, 123.17, 118.15, 117.42, 54.38, 39.23, 31.88, 31.78, 31.46, 31.45, 29.89, 29.55, 29.54, 265.30, 26.369, 24.26 ,14.09.
Embodiment 3
[0054] Example 3, 4,4'-(2,6-dibromobenzo[1,2-B:4,5-B']dithiophene-4,8-diyl)bis(1-2-octyl) Synthesis of dodecyl)-1H-1,2,3-triazole) (abbreviated as DBrTRI20)
[0055] Synthetic method is consistent with embodiment 1. A pale yellow powder (DBrTRI20, 2.0 g, 95%) was obtained.
[0056] The NMR data of the product are as follows: 1 H NMR (600MHz, CDCl 3 ),δ(ppm):8.05(s,2H),7.96(s,2H),4.44(d,4H),2.07(m,2H),1.36-1.25(m,64H),0.89-0.85(m, 12H). 13 C NMR (150MHz, CDCl 3 ), δ(ppm): 144.26, 138.51, 135.07, 126.12, 123.14, 118.17, 117.43, 54.38, 39.23, 31.91, 31.88, 31.45, 29.88, 29.65, 29.63, 29.59, 29.54, 29.30, 26.26.29 ,22.65,14.11,14.09.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com