Nonaqueous electrolyte solution, electrochemical device, lithium ion secondary cell, and module
A non-aqueous electrolyte and lithium-ion technology, applied in the direction of non-aqueous electrolyte batteries, secondary batteries, electrolytic capacitors, etc., can solve the problems of unrecorded impurities and reduced discharge capacity, and achieve excellent storage characteristics and high-voltage cycle characteristics Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0179] Hereinafter, although an Example and a comparative example are given and this invention is demonstrated more concretely, this invention is not limited to these Examples.
Synthetic example 1
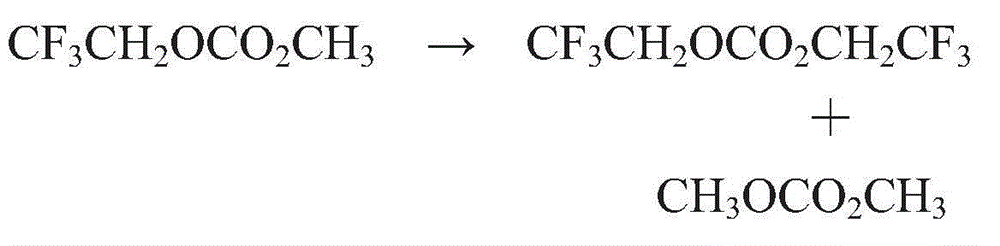
[0180] Synthesis Example 1CF 3 CH 2 OCO 2 CH 3 synthesis method
[0181] A 10 L four-necked flask was provided with a reflux tube and a dropping funnel to prepare a reaction apparatus. Then, add CF under ice bath 3 CH 2 OH (750 g; 7.5 moles), methyl chloroformate (708.8 g; 7.5 moles) and diglyme (700 mL) as solvent and stirred. Then, using a dropping funnel, triethylamine (758.3 g; 7.5 mol) was added while paying attention to heat generation. Triethylamine hydrochloride gradually precipitated, and the reaction solution changed to milky white.
[0182] After the reaction was completed, the reaction solution was washed with 1N aqueous HCl solution.
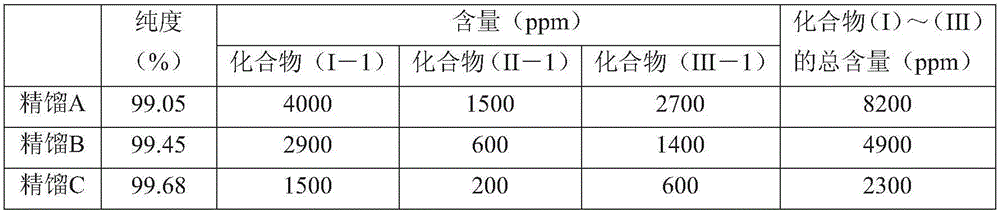
[0183] The organic layer separated after washing was rectified using a distillation refining column with 10 trays. About 5% of the initial distillation was discarded, and roughly equal samples were taken in the order of distillation, thus obtaining CF 3 CH 2 OH (compound (I-1)), CH 3 OH (compound (II-1)), CH 3 Rectifica...
Synthetic example 2
[0187] Synthesis Example 2 CF 3 CH 2 OCO 2 C 2 h 5 synthesis method
[0188] A 10 L four-necked flask was provided with a reflux tube and a dropping funnel to prepare a reaction apparatus. Then, add CF under ice bath 3 CH 2 OH (750 g; 7.5 moles), ethyl chloroformate (813.3 g; 7.5 moles) and diglyme (1250 mL) as solvent and stirred. Then, using a dropping funnel, triethylamine (758.3 g; 7.5 mol) was added while paying attention to heat generation. Triethylamine hydrochloride gradually precipitated, and the reaction solution changed to milky white. After the reaction was completed, the reaction solution was washed with 1N HCl aqueous solution.
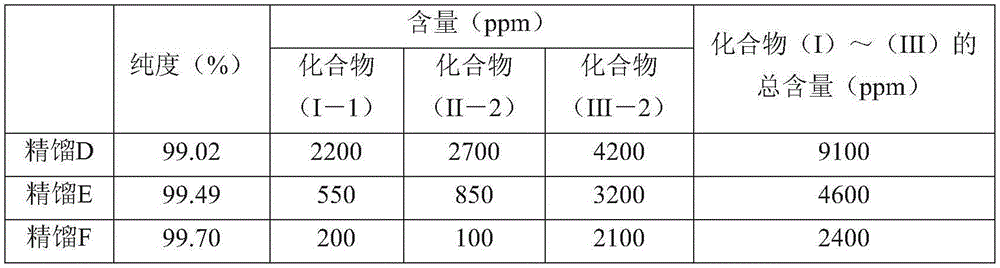
[0189] The organic layer separated after washing was rectified using a distillation refining column with 10 trays. About 5% of the initial distillation was discarded, and roughly equal samples were taken in the order of distillation, thus obtaining CF 3 CH 2 OH (compound (I-1)), C 2 h 5 OH (compound (II-2)), C 2 h 5 Recti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com