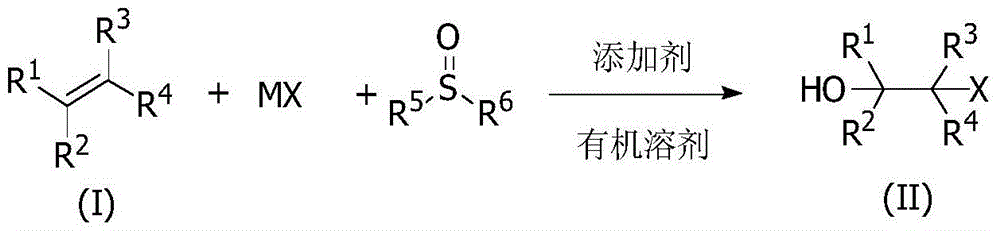
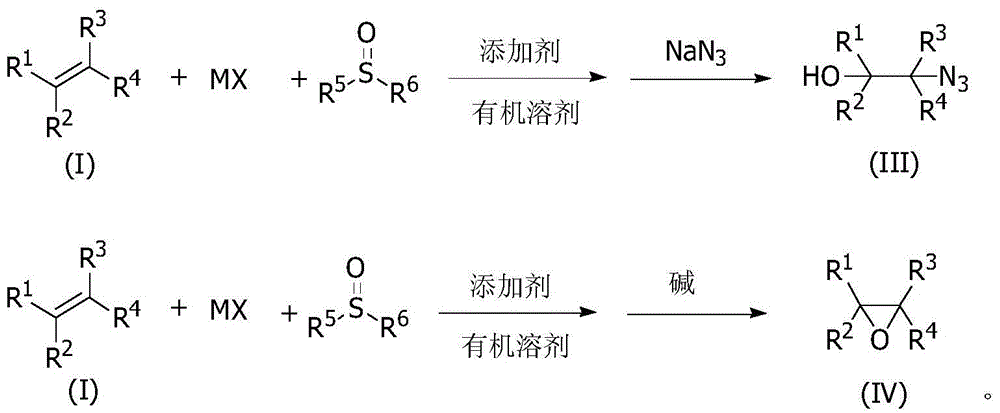
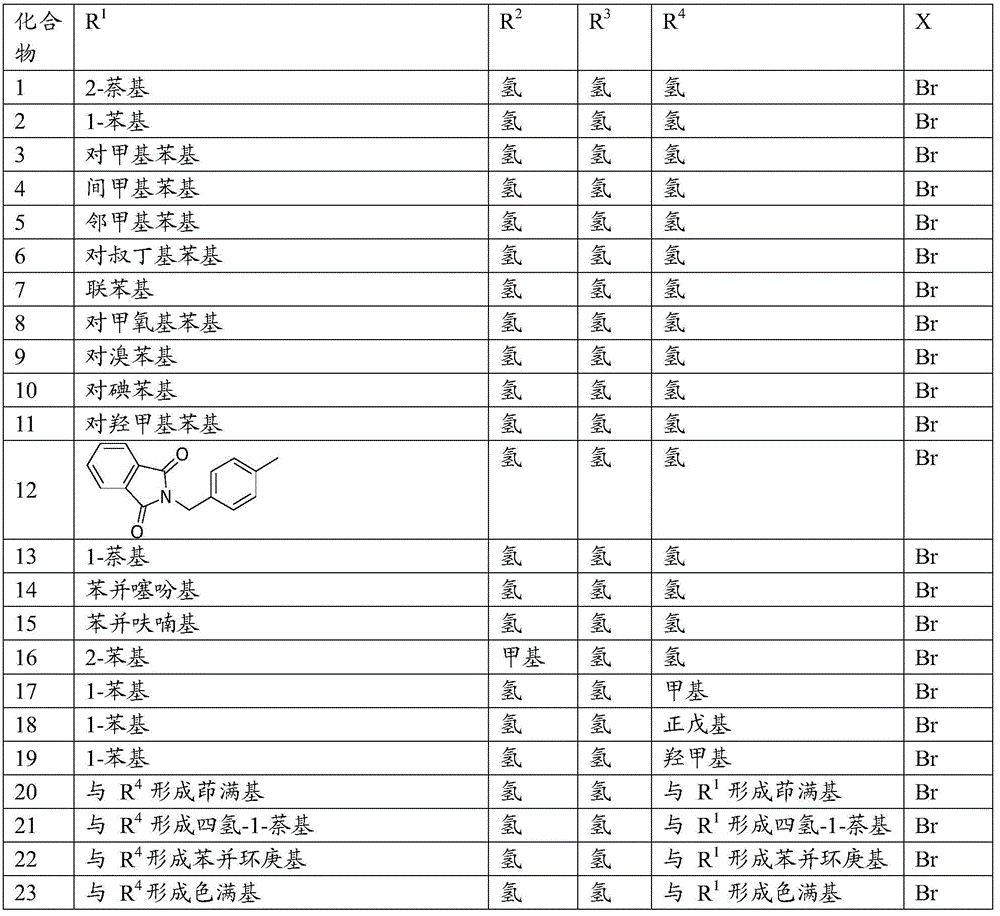
A Cheap and Efficient Synthesis Method of Halohydrin and Its Derivatives
A synthetic method and technology of haloalcohols, which are applied in organic chemical methods, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as high cost, low atomic economic rate, and difficult reaction operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Synthesis of 2-bromo-1-(2-naphthyl)-ethanol
[0028]
[0029] a): Take a 25mL Schlenk reaction tube, add 77mg of naphthalene ethylene, 101mg of 48% hydrobromic acid aqueous solution, 2mL of dimethyl sulfoxide, and stir at 60°C for 12 hours. Add 15 mL of ethyl acetate after the reaction to quench the reaction, add 5 mL of brine to wash, separate the organic phase, extract the aqueous phase with ethyl acetate for 3 times, combine the organic phases, and separate by column chromatography to obtain 2-bromo-1-(2- Naphthyl)-ethanol 105 mg, yield 84%.
[0030] b): Take a 25mL Schlenk reaction tube, add 77mg of naphthalene ethylene, 101mg of 48% hydrobromic acid aqueous solution, 2mL of dimethyl sulfoxide, and stir at 70°C for 12 hours. Add 15 mL of ethyl acetate after the reaction to quench the reaction, add 5 mL of brine to wash, separate the organic phase, extract the aqueous phase with ethyl acetate for 3 times, combine the organic phases, and separate by colu...
Embodiment 2
[0036] Example 2 Synthesis of 2-bromo-1-phenyl-ethanol
[0037]
[0038] Take a 25mL Schlenk reaction tube, add 52mg of styrene, 101mg of 48% hydrobromic acid aqueous solution, and 2mL of dimethyl sulfoxide, and stir at 60°C for 12 hours. After the reaction was completed, 15 mL of ethyl acetate was added to quench the reaction, and 5 mL of brine was added for washing. The organic phase was separated, and the aqueous phase was extracted three times with ethyl acetate. The organic phases were combined and separated by column chromatography to obtain 2-bromo-1-phenyl- Ethanol 72 mg, yield 71%.
[0039] 1 H NMR (400MHz, CDCl 3 )δ7.38–7.31(m,5H),4.92(dt,J=8.9,3.1Hz,1H),3.63(dd,J=10.5,3.3Hz,1H),3.56–3.51(m,1H),2.68 (d,J=3.2Hz,1H); 13 C NMR (100MHz, CDCl 3 ) δ 140.2, 128.7, 128.4, 125.9, 73.8, 40.2.
Embodiment 3
[0040] Example 3 Synthesis of 2-bromo-1-(4-methyl-phenyl)-ethanol
[0041]
[0042] Take a 25mL Schlenk reaction tube, add p-methylstyrene 59mg, 48% hydrobromic acid aqueous solution 101mg, dimethyl sulfoxide 2mL, and stir at 60°C for 12 hours. After the reaction was completed, 15 mL of ethyl acetate was added to quench the reaction, and 5 mL of brine was added for washing. The organic phase was separated, and the aqueous phase was extracted 3 times with ethyl acetate. The organic phases were combined and separated by column chromatography to obtain 2-bromo-1-(4- Methyl-phenyl)-ethanol 97 mg, yield 90%.
[0043] 1 H NMR (400MHz, CDCl 3 )δ7.25(d,J=8.3Hz,2H),7.18(d,J=7.9Hz,2H),4.88–4.86(m,1H),3.60(dd,J=10.4,3.4Hz,1H), 3.52(dd,J=10.4,3.4Hz,1H),2.64(d,J=3.0Hz,1H),2.35(s,3H); 13 C NMR (100MHz, CDCl 3 ) δ 138.2, 137.3, 129.3, 125.9, 73.6, 40.2, 21.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com