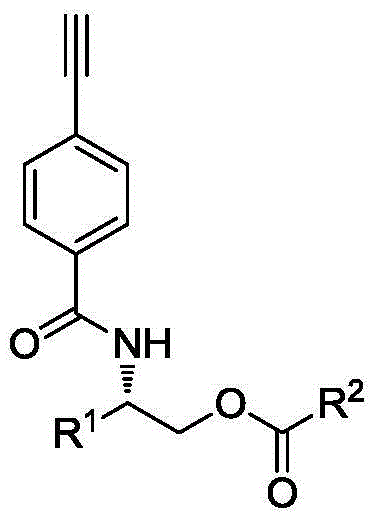
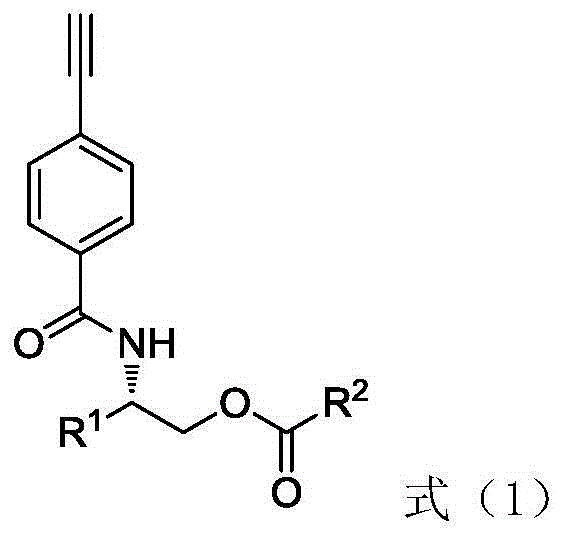
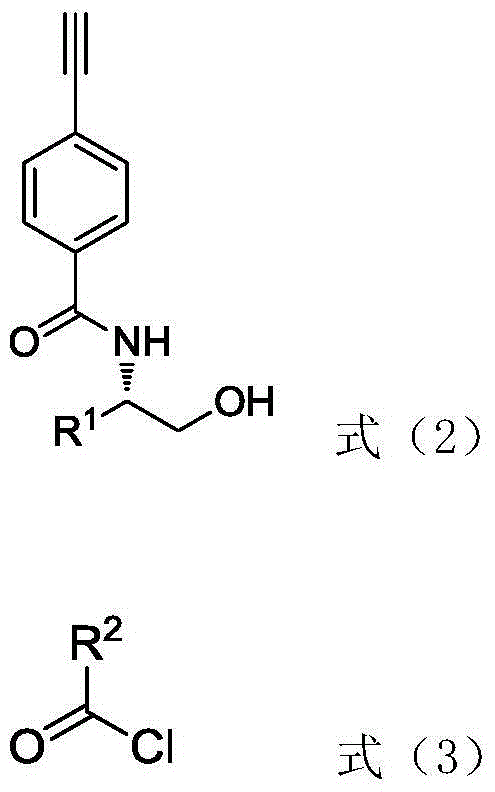
Phenylacetylene derivative with ester group on lateral base band and preparation, polymerization and application methods
A technology of derivatives and phenylacetylene, which is applied in the field of liquid chromatography chiral stationary phase compounds, can solve problems such as potential safety hazards, and achieve the effects of low cost, short preparation cycle and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] Furthermore, the present invention provides a method for preparing a chiral stationary phase from the above-mentioned helical polyphenylene vinylene derivatives with ester groups in side groups, and the chiral stationary phase prepared therefrom. The preparation method includes: using an organic solvent as a coating solvent, coating the helical polyphenylene vinylene derivative synthesized above on aminopropyl silica gel to prepare a coating-type high performance liquid chromatography chiral stationary phase.
[0064] The coating solvent is one of chloroform, dichloromethane, methanol, acetone, tetrahydrofuran, N,N-dimethylformamide, N,N-dimethylacetamide, dimethyl sulfoxide, or Various.
Embodiment 1
[0067] (1) A novel optically active phenylacetylene derivative with a butyl benzoate group—(S)-2-(4-ethynylphenylcarbonylamino)-3-methylbenzoic acid butyl (PA-A- The synthetic method of Val-Ben) is: under normal temperature and normal pressure, take by weighing 1.0345g N-(4-ethynylbenzoyl)-L-valinol, according to N-(4-ethynylbenzoyl)- L-valinol, benzoyl chloride, triethylamine molar ratio is 1:1:1 Take benzoyl chloride and triethylamine dissolved in 45mL tetrahydrofuran for reaction, the reaction is carried out under the protection of nitrogen, the reaction temperature is 25 ℃ , The reaction time is 12h. After the reaction, the reaction solution was extracted by rotary evaporation to remove the solvent. The solvent used in the extraction method is dichloromethane / water. It is then purified by column chromatography. The eluent used in column chromatography was chloroform / ethyl acetate (20 / 1, V / V). The product yield was 1.3728 g, and the yield was 91.52%. Molecular formula ...
Embodiment 2
[0075] (1) A novel optically active phenylacetylene derivative with a butyl propionate group—(S)-2-(4-ethynylphenylcarbonylamino)-3-methylpropionate butyl (PA-A- The synthetic method of Val-Pro) is: under normal temperature and normal pressure, take by weighing 0.80g N-(4-ethynylbenzoyl)-L-valinol, according to N-(4-ethynylbenzoyl)- The molar ratio of L-valinol, L-propionyl chloride, and triethylamine is 1:2:5, and benzoyl chloride and triethylamine are dissolved in 40 mL of tetrahydrofuran for reaction. The reaction is carried out under nitrogen protection, and the reaction temperature is 35 °C, the reaction time is 24h. After the reaction, the reaction solution was extracted by rotary evaporation to remove the solvent. The solvent used in the extraction method is dichloromethane / water. It is then purified by column chromatography. The eluent used in column chromatography was chloroform / ethyl acetate (15 / 1, V / V). The product yield was 0.8187 g, and the yield was 82.36%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com