Derivative of chiral tetrahydroisoquinoline or salt thereof, preparation method and use thereof
A technology for tetrahydroisoquinoline and chiral compounds, which is applied in the field of chiral tetrahydroisoquinoline derivatives or their salts and their preparation, and can solve problems such as poor absorption and irregularities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] Preparation method of chiral compound
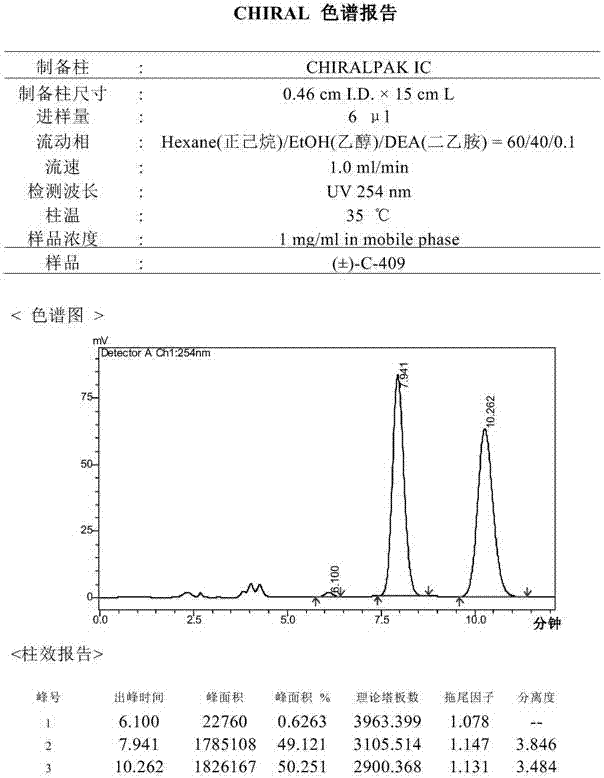
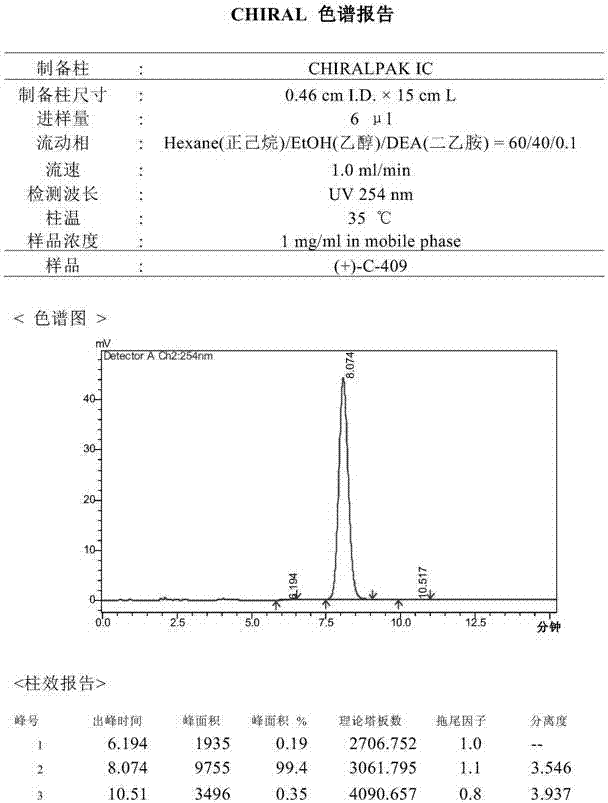
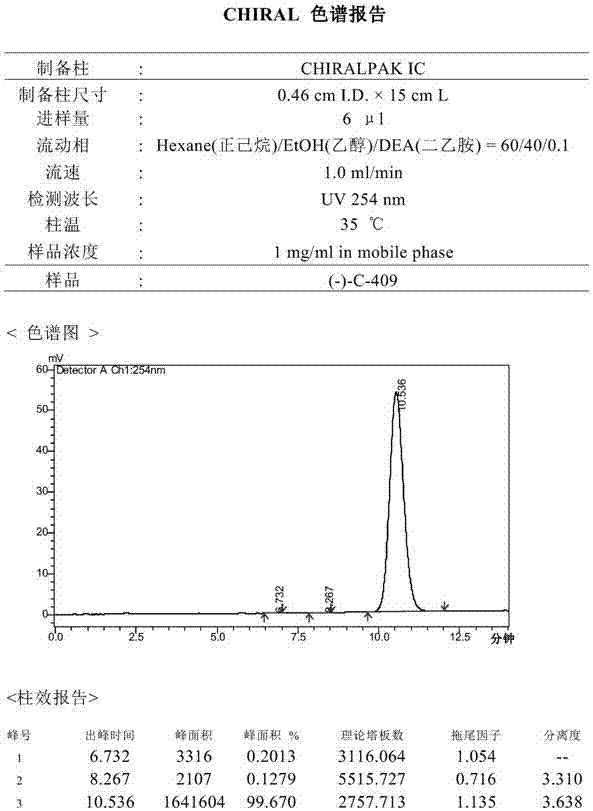
[0031] The inventors prepared and separated the optical isomers in the isoquinoline compound through a chiral preparation column, and obtained (+)-C-409 and (-)-C-409 of chiral purity respectively:
[0032]
[0033] The method of chiral separation and preparation is not strictly required, as long as the (+)-C-409 and (-)-C-409 in the racemic (±)-C-409 are separated according to the conventional method of chiral separation and preparation .
[0034] Separation of (+)-C-409 and (-)-C-409 in racemic (±)-C-409 requires some separation method research.
[0035] Choose the chiral column CHIRALPAK ADHs (0.46cm I.D.×15cm L): (1) The mobile phase is n-hexane:isopropanol:diethylamine=70:30:0.1, the flow rate is 1ml / min, the column temperature is 35.0℃, and the Sample volume (4ul), sample concentration (3.2mg / ml), (+)-C-409 and (-)-C-409 are partially separated; (2) The mobile phase is n-hexane:ethanol:diethylamine=75: 25:0.1, flow rat...
Embodiment 1
[0059] (+)-1-(3-Methanesulfonamidobenzyl)-6-methoxy-7-benzyloxy-1,2,3,4-tetrahydroisoquinoline ((+)-C-409 ) and (-)-1-(3-methanesulfonamidobenzyl)-6-methoxy-7-benzyloxy-1,2,3,4-tetrahydroisoquinoline ((-)-C -409) preparation
[0060] Chiral preparative liquid phase method: Instrument: K-prep HPLC machine UV detector from YMC Company, chromatographic column: chiralpak IC column, 3cm I.D.*25cm Length, mobile phase: Hexane / Ethanol=50 / 50(v / v), The flow rate is 20ml / min, the temperature is 35°C, and the wavelength is 254nm.
[0061] 734 mg of the raw material (racemic (±)-C-409) was prepared into a mobile phase solution with a concentration of 6 mg / ml. Each injection is 6ml, that is, a sample loading is 36mg. Prepared by the above-mentioned chiral preparative liquid phase method, and collected the effluents containing (+)-C-409 and (-)-C-409 components respectively. The operation was repeated with 6 ml injections until the racemic C-409 stock solution was exhausted. The (+)-C-...
Embodiment 2
[0064] (+)-1-(3-Methanesulfonamidobenzyl)-6-methoxy-7-benzyloxy-1,2,3,4-tetrahydroisoquinoline ((+)-C-409 ) hydrochloride and (-)-1-(3-methanesulfonamidobenzyl)-6-methoxy,7-benzyloxy-1,2,3,4-tetrahydroisoquinoline ((- )-C-409) Preparation of hydrochloride
[0065] Dissolve 301 mg of (+)-C-409 in 10 ml of methanol, add 0.3 ml of concentrated hydrochloric acid dropwise, shake until completely dissolved, evaporate the solvent with a rotary evaporator to obtain a solid product, and transfer it to a sample bottle to obtain (+) C-409 Hydrochloride 285mg. The specific rotation of (+)-C-409 hydrochloride is: [α]2D5=+20.6 (C1, 1% methanol hydrochloride). Use the following HPLC conditions for detection: Chiral column CHIRALPAK IC (0.46cm I.D.×15cm L), mobile phase is n-hexane:ethanol:diethylamine=60:40:0.1, flow rate is 1ml / min, detection wavelength is 220nm, column temperature At 35.0°C, the sample was dissolved in mobile phase, the injection volume (5ul), and the sample purity was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com