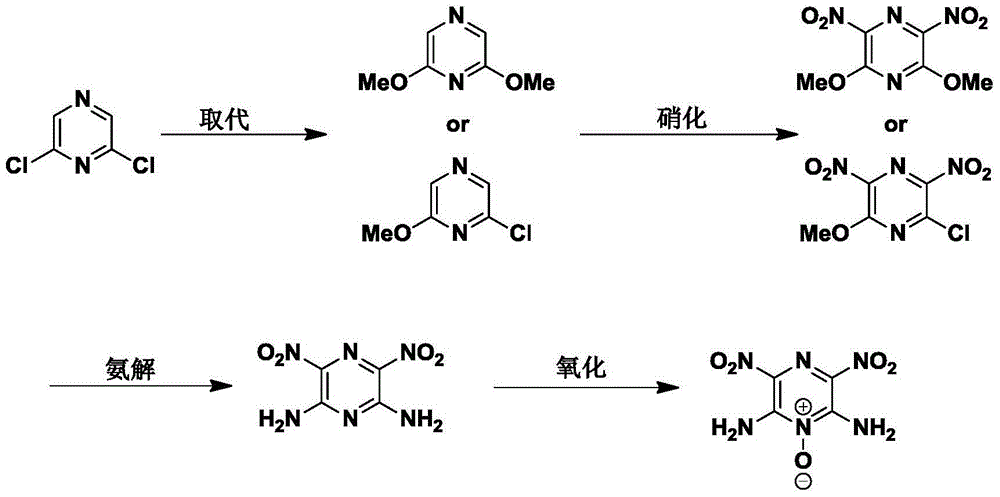
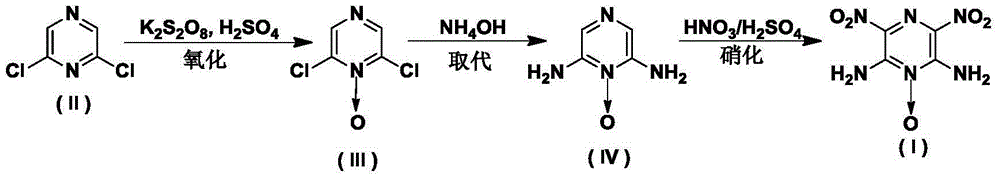
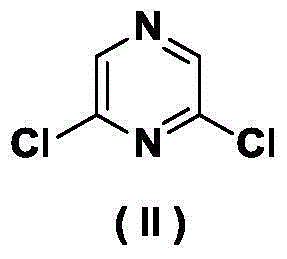
Synthesis method of 2,6-diamido-3,5-dinitropyrazine-1-oxide
A technology of dinitropyrazine and diaminopyrazine, applied in the direction of organic chemistry and the like, can solve the problems of strong solvent volatility, difficult recovery, high price, etc., and achieves the advantages of cheap and environmentally friendly solvents, shortened synthesis process, and reduced manufacturing costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1. the synthesis of formula (III) compound 2,6-dichloropyrazine-1-oxide
[0025]
[0026] At 0°C, add 1.5g (10mmol) (II) into 10mL 95% concentrated sulfuric acid and stir, add 0.3g (1.1mmol) of potassium persulfate every 10 minutes, and add 3g (11mmol) of potassium persulfate in total. After the reaction system was raised to room temperature and stirred for 48 h, it was poured into ice, extracted with ethyl acetate (4×50 mL), washed with 10 mL of saturated brine, dried with 1.4 g (10 mmol) of anhydrous sodium sulfate, filtered, and evaporated to dryness to obtain the formula ( III) Compound 2,6-dichloropyrazine-1-oxide, yield 88.0%.
Embodiment 2
[0027] Embodiment 2. the synthesis of formula (III) compound 2,6-dichloropyrazine-1-oxide
[0028] At 0°C, 1.5 g (10 mmol) (II) was added into 10 mL of 95% concentrated sulfuric acid and stirred, and 3 g (11 mmol) of potassium persulfate was added rapidly. After the reaction system was raised to room temperature and stirred for 48 h, it was poured into ice, extracted with ethyl acetate (4×50 mL), washed with 10 mL of saturated brine, dried with 1.4 g (10 mmol) of anhydrous sodium sulfate, filtered, and evaporated to dryness to obtain the formula ( III) Compound 2,6-dichloropyrazine-1-oxide, yield 85.0%.
Embodiment 3
[0029] Embodiment 3. the synthesis of formula (III) compound 2,6-dichloropyrazine-1-oxide
[0030] At 0°C, add 1.5g (10mmol) (II) into 10mL 95% concentrated sulfuric acid and stir, add 0.3g (1.1mmol) of potassium persulfate every 10 minutes, and add 3g (11mmol) of potassium persulfate in total. After the reaction system was raised to room temperature and stirred for 24 hours, 0.6 g (2.2 mmol) of potassium persulfate was added, and the reaction was continued for 24 hours. Poured into ice, extracted with ethyl acetate (4×50mL), washed with 10mL of saturated brine, dried with 1.4g (10mmol) of anhydrous sodium sulfate, filtered, and evaporated to dryness to obtain formula (III) compound 2,6-dichloro Pyrazine-1-oxide, yield 91.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com