Trithio ester compounds containing quantitative sulfate ethyl sulfone group, and synthesis method and application thereof
A technology of sulfate ethyl sulfone and trithioester, which is applied in the fields of dyeing, textiles and papermaking, can solve the problems of no chemical bond between latex and natural fiber, low dispersion and stability of latex particles, etc., and achieve good application Foreground, wide range of applicable monomers, good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of monomer containing sulfate ethylsulfone groups (monomer A):
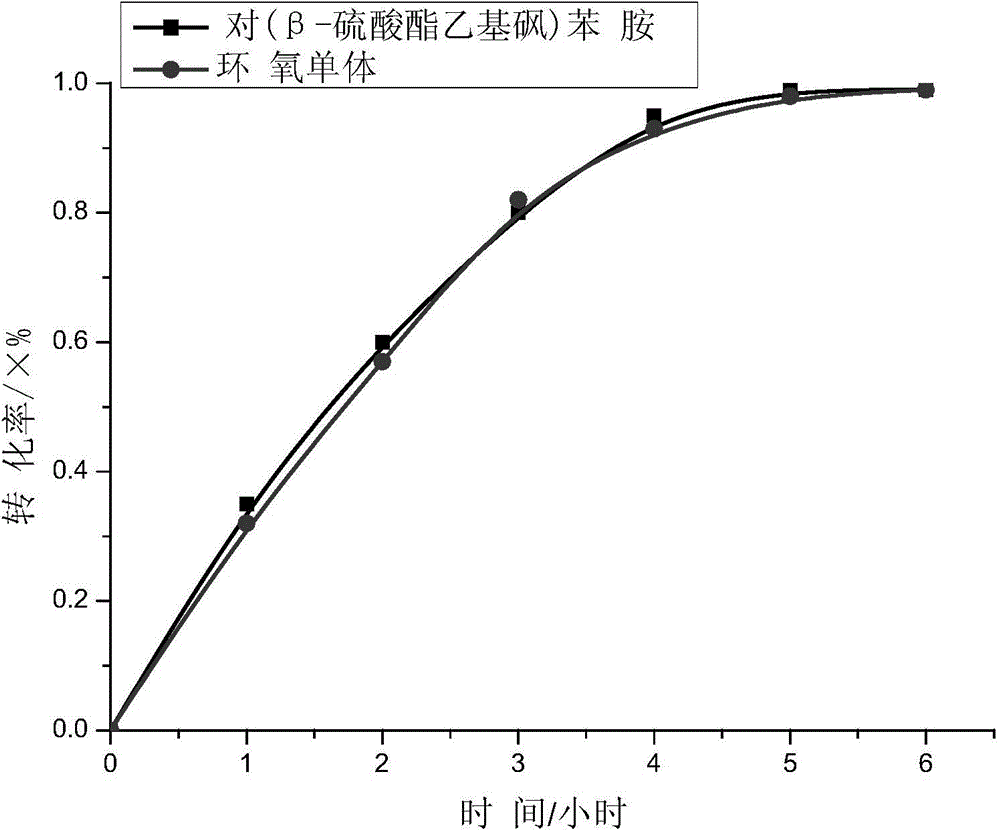
[0055] Weigh 2.0g of p-(β-sulfate ethylsulfone) aniline (Formula 6) and add it to 30g of DMF, stir until it dissolves, then transfer the solution into a 100mL three-neck flask, raise the temperature to 80°C, and add 0.9763g of epoxy The monomer (formula 9) and 0.5mL formic acid were reacted for 5 hours, the temperature was lowered to stop the reaction, and the monomer (formula 10) containing sulfated ethyl sulfone groups, ie, monomer A, was obtained. The curve of reaction conversion rate as a function of time is shown as figure 1 shown.
[0056] After reacting for 5 hours, the conversion rate of (β-sulfate ethyl sulfone) aniline and the epoxy monomer with the structure of formula 9 is close to 100%. From this, the monomer A can be calculated, which is the structure of formula 10.
[0057]
Embodiment 2
[0059] Preparation of amphiphilic structure macromolecular RAFT reagent containing "sulfate ethyl sulfone group", the structural formula is as formula III.
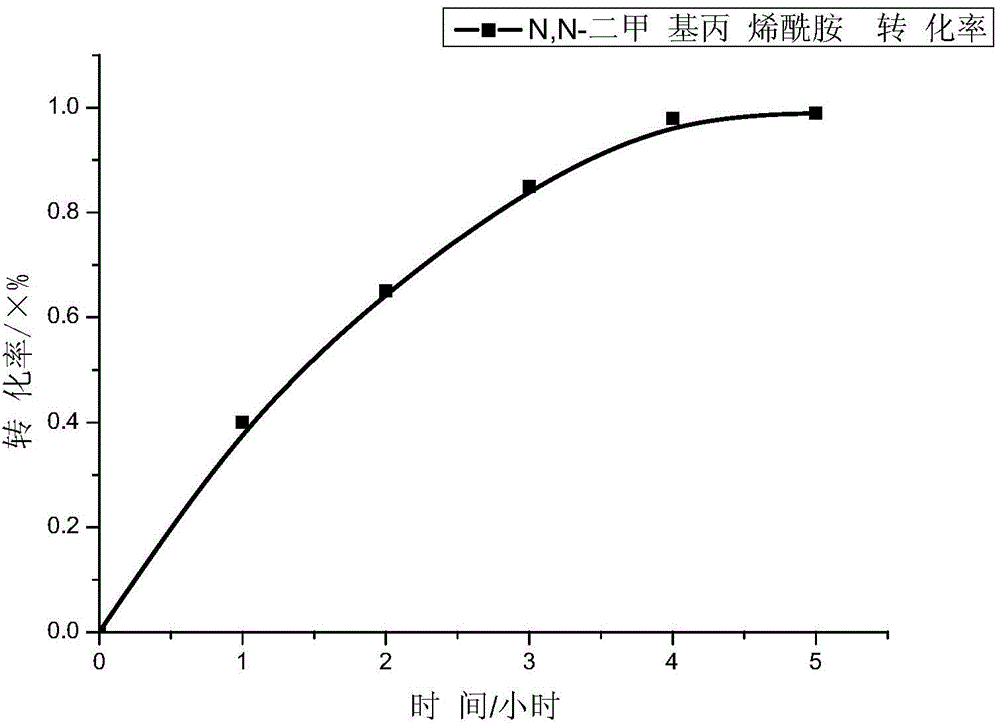
[0060] Put the following reagents into a 250mL three-necked flask equipped with a reflux condenser, nitrogen inlet and feed port: 30 grams of dioxane, 1.50 grams of small molecule RAFT reagent (when n=12, the corresponding structural formula of formula II), azobis Cyanovaleric acid 0.12 g and N,N-dimethylacrylamide 12.73 g. After passing high-purity (99.99%) nitrogen gas through the above device for 1 hour, it was immersed in a water bath at 80° C. under magnetic stirring, and reacted for 5 hours. The curve of reaction conversion rate as a function of time is shown as figure 2 shown.
[0061] Continue to add 21 grams of dioxane, 0.12 grams of azobiscyanovaleric acid, the monomer (i.e., monomer A) 18.13 of the formula 10 structure prepared in Example 1 containing ethylsulfone sulfate group in the there-necked flask gra...
Embodiment 3
[0065] The preparation of the macromolecular RAFT reagent with amphiphilic structure containing "sulfate ethyl sulfone group", the structural formula is as formula IV.
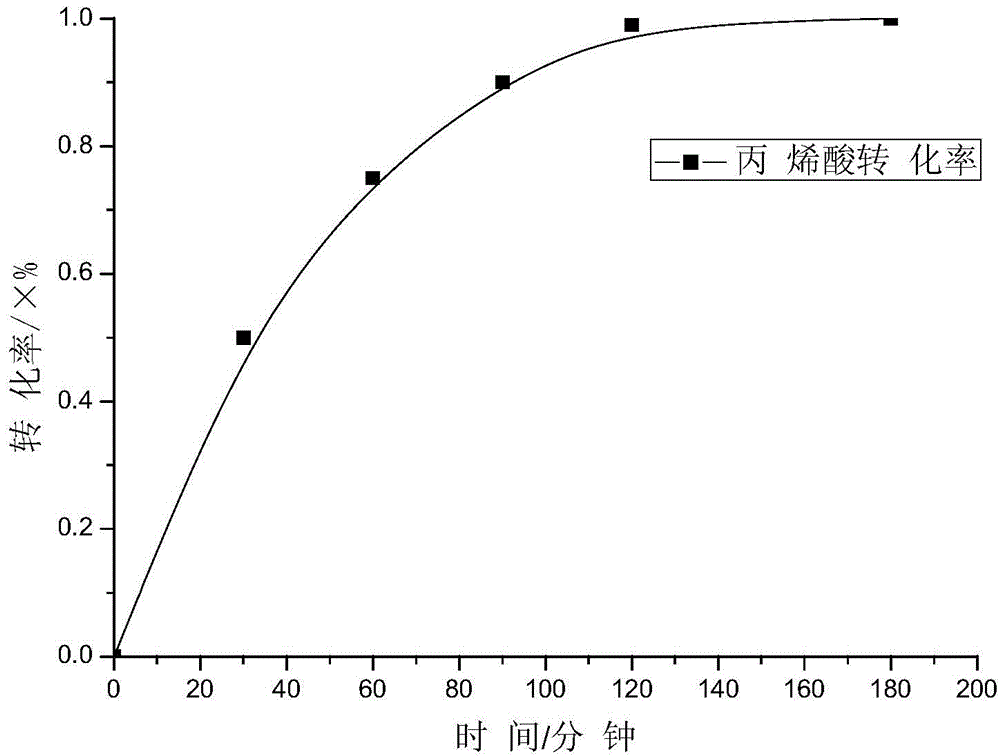
[0066] Put the following reagents into a 250mL three-necked flask equipped with a reflux condenser, a nitrogen inlet, and a feed port: 21 grams of dioxane, 1.50 grams of small molecule RAFT reagent (when n=12, the corresponding structural formula of formula II), azobis Cyanovaleric acid 0.12 g and acrylic acid 9.25 g. After passing high-purity (99.99%) nitrogen gas through the above device for 1 hour, it was immersed in a water bath at 80° C. for 2 hours to react. The curve of reaction conversion rate as a function of time is shown as image 3 shown.
[0067] Continue to add 21 grams of dioxane, 0.12 grams of azobiscyanovaleric acid, the monomer (i.e., monomer A) 18.13 of the formula 10 structure prepared in Example 1 containing ethylsulfone sulfate group in the there-necked flask gram, continued to react i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com