Crosslinkable high dielectric norbornene copolymer, norbornene crosslinked polymer and preparation method thereof
A norbornene and copolymer-like technology, applied in the field of high dielectric polymer preparation, can solve the problems of being susceptible to solvents, brittleness, corrosion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The preparation method of the present invention comprises the following steps:
[0062] (1) Combine endo-N-3,5-bistrifluoromethylbiphenylnorbornene pyrrolidine or exo-N-3,5-bistrifluoromethylbiphenylnorbornenepyrrolidine with exo- N-ethyl cinnamate-7-oxanorbornene imide and Grubbs catalyst were respectively dissolved in an organic solvent, frozen in liquid nitrogen, evacuated and filled with nitrogen, and thawed, and the operation was repeated 3 times. After preheating for 10 minutes, add Grubbs catalyst to endo- / exo-N-3,5-bistrifluoromethylbiphenylnorbornene pyrrolidine, stir at 30°C for 50 minutes, and exo-N-cinnamon under nitrogen protection An organic solvent solution of ethyl acetate-7-oxanorbornene imide was added thereto, and stirring was continued for 1 h.
[0063] Wherein, the catalyst addition amount is endo-N-3,5-bistrifluoromethyl biphenyl norbornene pyrrolidine or exo-N-3,5-bis trifluoromethyl biphenyl norbornene pyrrolidine 0.02 of the molar mass.
[00...
Embodiment 1
[0070] Embodiment 1: endo, the preparation of exo-configuration norbornene block copolymer
[0071] In a 25mL Schlenk reaction tube, add endo-N-3,5-bistrifluoromethylbiphenylnorbornene pyrrolidine (0.423g, 1mmol), vacuumize and replace with high-purity nitrogen for 3 times, add 3mL dichloromethane to dissolve completely. In another 25mL Schlenk reaction tube, add exo-N-ethyl cinnamate-7-oxanorbornenimide (0.339g, 1mmol), vacuumize and replace with high-purity nitrogen for 3 times, add 7.5mL di Chloromethane to dissolve completely. Under nitrogen atmosphere, weigh Grubbs first generation catalyst RuCl 2 (PCy 3 ) 2(CHPh) (16.5 mg, 0.02 mmol) was placed in the third Schlenk tube, after vacuuming and nitrogen replacement for 3 times, 2 mL of dichloromethane was added. The reaction system was frozen with liquid nitrogen, and vacuumized and filled with nitrogen. After repeating the cycle 3 times, thawed, preheated at 30°C for 10 minutes, and then quickly added the catalyst solu...
Embodiment 2
[0074] Embodiment 2: exo, the preparation of exo-configuration norbornene block copolymer
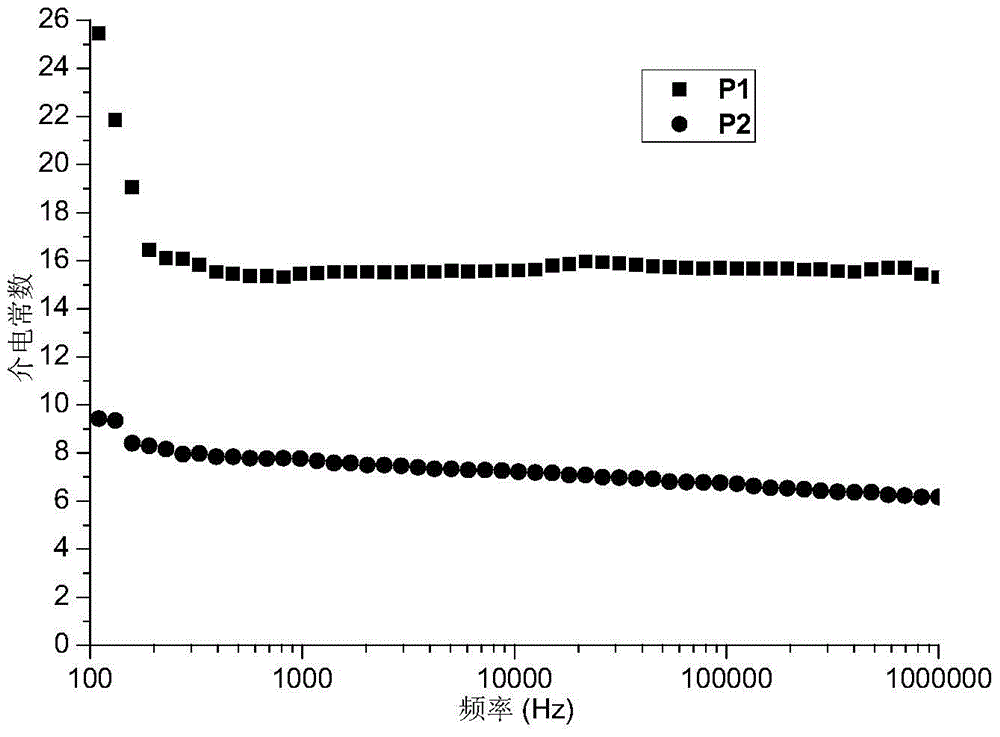
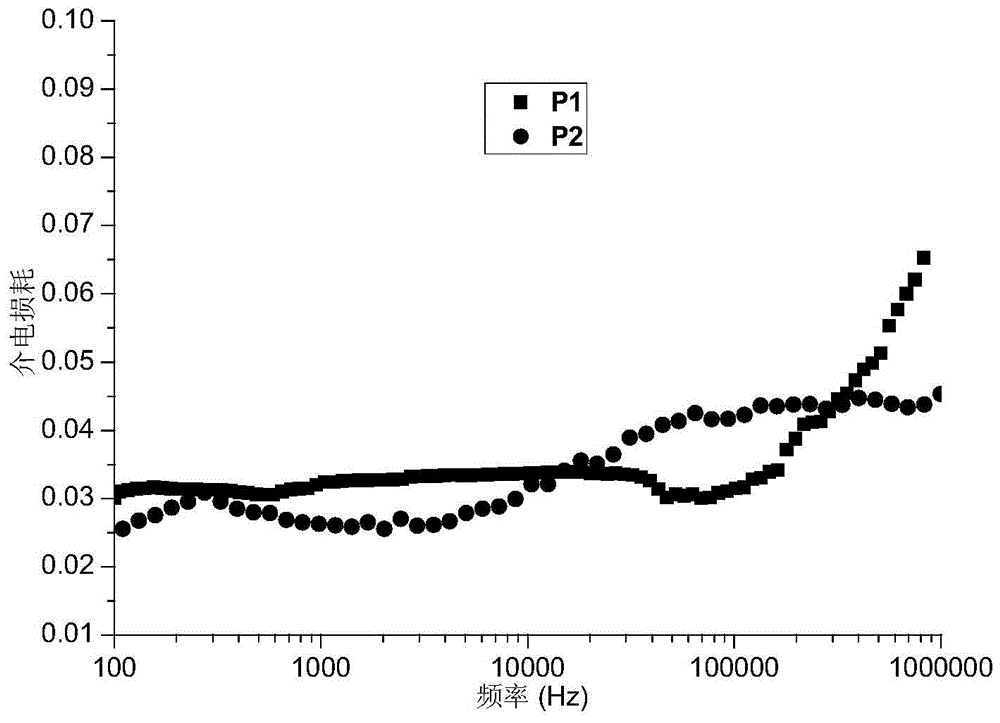
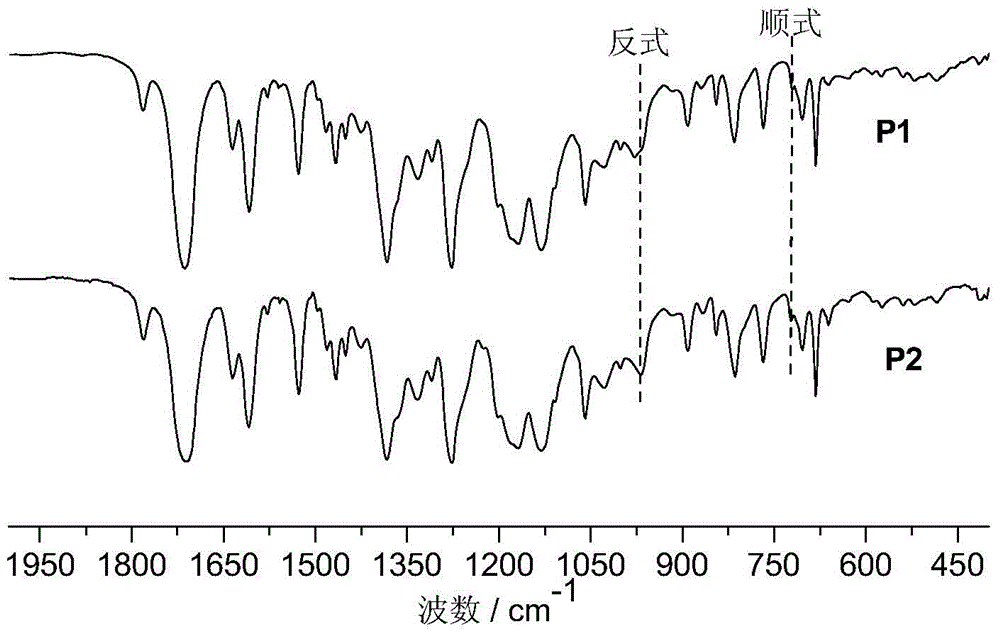
[0075] The high mesofunctional monomer in this example is exo-N-3,5-bistrifluoromethylbiphenylnorbornene pyrrolidine, and other reaction conditions and steps are the same as those in Example 1. 0.747 g of the polymer product was obtained with a yield of 98%. The resulting product is a copolymer of exo-N-3,5-bistrifluoromethylbiphenylnorbornene pyrrolidine and exo-N-ethyl cinnamate-7-oxanorbornene imide, and its structure As shown in formula (P2):
[0076]
[0077] 1 H NMR (500MHz, CDCl 3 , ppm): δ=7.97 (m, o-ArH-CF 3 ), 7.74-7.70 (m, p-ArH-CF3), 7.70-7.68 (d, ArH-CH=CH), 7.59-7.29 (m, m-ArH-NCH 2 +ArH-CH=CH), 6.84-6.58 (m, o-ArH-NCH 2 ), 6.42-6.33 (m, ArH-CH=CH), 6.05-5.30 (m, CH=CH on polymer chain), 5.1-4.9 (s, CHOCH), 4.54-4.24 (t, CH 2 OCO), 3.9-3.7(t, NCH 2 ), 3.5-3.1 (m, ArNCH 2 +CHCONCOCH), 2.8-2.3(m, CHCHCH 2 N+CHCH 2 N), 1.5-1.2(d, CHCH 2 CH); 13 C NMR (125MHz, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com