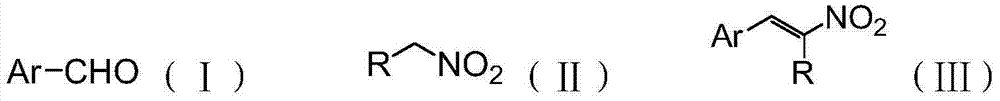Alpha, beta-nonsaturated nitroolefin compound eco-friendly synthesis method
A nitroolefin and green synthesis technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of multi-step, good yield, and low yield, etc., to achieve atomic efficiency and reduce impact , the effect of cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
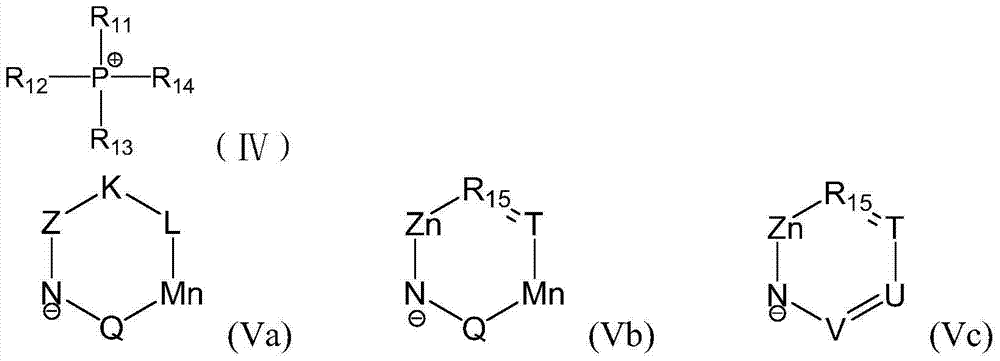
[0054] Preparation of the first step ionic liquid
[0055] Take tetrabutylphosphorus hydroxide aqueous solution (mass concentration 40%) (6.9g, 25mmol) and equimolar amount of imidazole (0.7g, 25mmol), stir at room temperature for 48h, remove water and dry to obtain a colorless transparent liquid, vacuum at 80°C Dry for 12h to obtain ionic liquid [P(n-Bu) 4 ][Im], the structural formula is as follows:
[0056]
[0057] The second step Henry reaction
[0058] Functionalized ionic liquid [P(C 2 h 5 ) 4 ][Im] (1.0g, 3.1mmol) and 1mL of water were added to a 50mL microblogging quartz tube, stirred at room temperature until homogeneous, nitroethane (0.1g, 2.5mmol) and benzaldehyde (0.26g, 2.5mmol) were sequentially Add it into the reaction vessel, seal it with a microwave polytetrafluoroethylene cover, stir for 30 minutes under microwave conditions (temperature 60°C, power 400W), and cool to room temperature. Take a small amount of the reaction mixture to do 1 H NMR chara...
Embodiment 2-5
[0063] Microwave temperature is as described in Table 1, other reaction reaction conditions: functionalized ionic liquid [P(n-Bu) 4 ] [Im], water consumption, reaction time, reactant consumption and product yield calculation are reacted with the henry among the embodiment 1.
[0064] Table 1
[0065]
Embodiment 6-11
[0067] The reaction time is described in Table 2, other reaction reaction conditions: functionalized ionic liquid [P(n-Bu) 4 ] [Im], water consumption, reaction time, reactant consumption and product yield calculation are reacted with the henry among the embodiment 1. .
[0068] Table 2
[0069]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com