Vitamin D2 glucoside analogue, synthesis and application thereof
A technology of vitamins and analogs, applied in the field of medicinal chemistry, to achieve good inhibitory effect, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In conjunction with the synthetic route of formula (I), the synthesis of compound 1:
[0039] Add vitamin D to a 100mL three-neck flask 2 (0.50g, 1.26mmol) and 50mL of ethanol were placed in a low-temperature circulation tank, and the temperature was slowly lowered to below -20°C, then potassium permanganate (0.80g, 5.04mmol) was dissolved in 20mL of water, and added dropwise to the reaction system Medium (control the temperature not to exceed -20°C). After the dropwise addition, keep the temperature between -30°C and -20°C, and continue to stir and react for 2.5h. Then the temperature was raised slowly, and the temperature was controlled between -17°C--15°C to continue the reaction for 1.5h. After that, the temperature was naturally raised to room temperature, and then slowly heated to 45° C., and the reaction was continued for 10 minutes. Then TLC monitored the reaction (V ethyl acetate: V petroleum ether = 1:1). After the reaction, it was cooled to room temperatu...
Embodiment 2
[0041] In conjunction with the synthetic route of formula (I), the synthesis of compound 2:
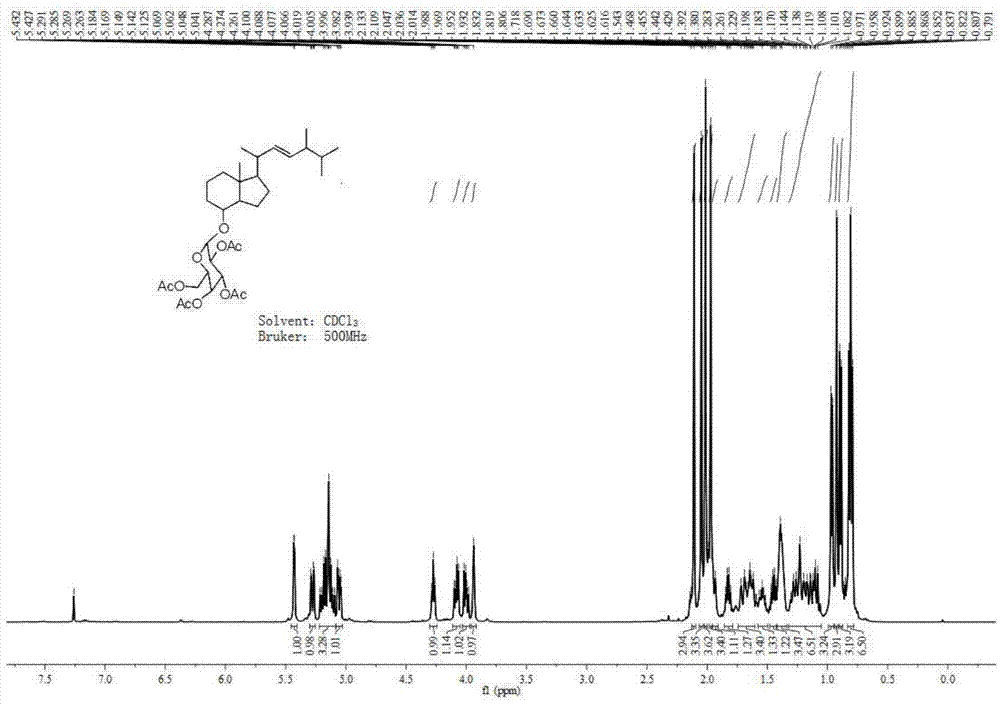
[0042] Compound 1 (0.10 g, 0.23 mmol), lead tetraacetate (0.13 g, 0.279 mmol) and 10 mL of toluene were added to a 50 mL two-necked flask respectively. Then nitrogen protection was introduced, and the stirring reaction was continued for 5 h at room temperature. The reaction was monitored by TLC (V ethyl acetate: V petroleum ether = 1:4). After the reaction is complete, filter and concentrate under reduced pressure to obtain the crude compound. This step does not require purification, and proceeds directly to the next step.
Embodiment 3
[0044] In conjunction with the synthetic route of formula (I), the synthesis of compound 3:
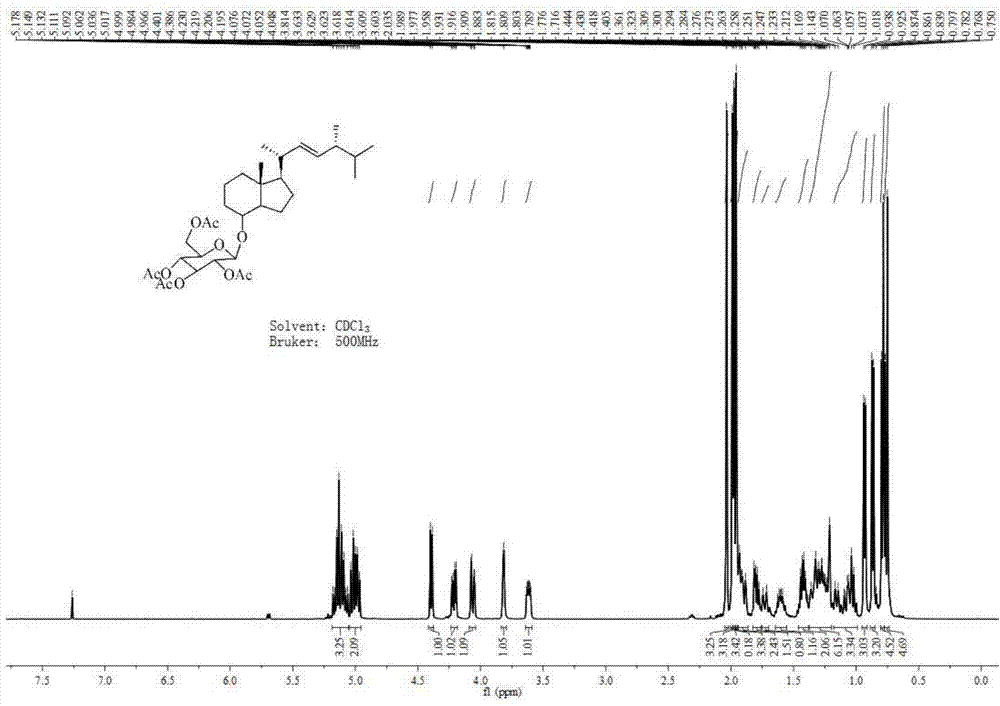
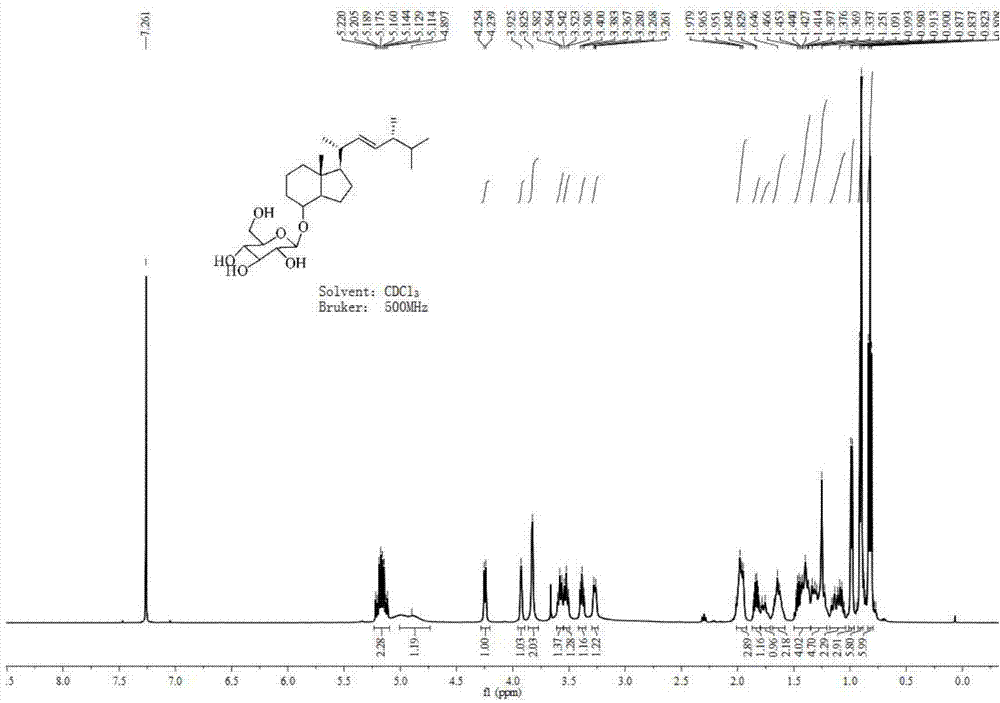
[0045] Concentrate and dry the crude product obtained in the previous step, weigh it (0.19 g, 0.68 mmol), dissolve it in 10 mL of redestilled toluene, and add 5 mL of ethanol. The reaction was placed in a low-temperature circulation tank, and the temperature was slowly lowered to -10°C. Sodium borohydride (0.10 g, 1.45 mmol) was then added slowly with stirring. After the addition, continue to stir the reaction at low temperature for 15 minutes, then place the reaction at room temperature, and continue to stir the reaction for 2 hours. The progress of the reaction was monitored by TLC (V ethyl acetate: V petroleum ether = 1:10). After the reaction was complete, an appropriate amount of water was slowly added at low temperature to quench the reaction. Then, 100 mL of ethyl acetate and 100 mL of water were added, and after shaking and separating, the organic layer was separated. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com