Preparation method for (2'R)-2'-deoxy-2'-fluoro-2'-methyluridine
A technology of methyl ureaside and dibenzoyl ureaside, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve problems such as the utilization rate of chemical atoms with short route and cost, and achieve low product cost. , The chemical synthesis route is short, and the purity is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
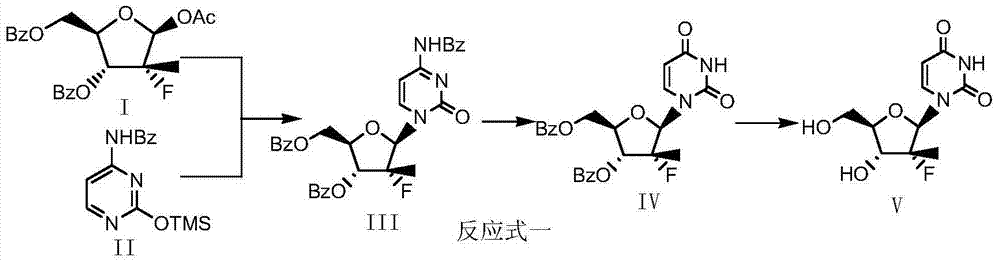
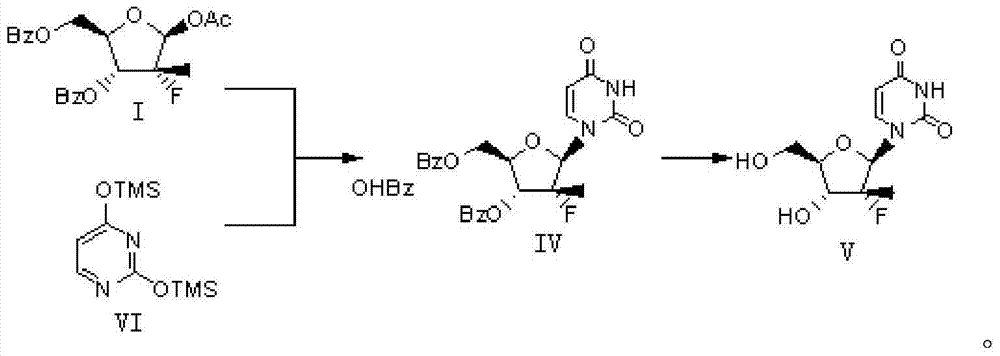
[0020] Step 1: Preparation of Dibenzoyluridine (Formula IV)
[0021] In a 500ml four-necked flask, put 20.8g of protected fluororibose (formula I), 100ml of chlorobenzene, and 14.1g of ditrimethylsilica protected uracil (VI), stir, and the reaction solution will be heated to the range of 60-80℃ Slowly add 52.0g of tin tetrachloride, after about 30min, the reaction solution is dissolved and clarified. After stirring and reacting at 60-80℃ for 30min, a large amount of solid precipitates out. After the reaction is kept at this temperature for 17-18h, sample, HPLC It was detected that the reaction of the raw materials was basically complete, the temperature was lowered to room temperature (20°C), 150ml of dichloromethane was added, and the mixture was stirred and diluted to obtain diluent A.
[0022] In another 500ml four-neck flask, add 52g of sodium bicarbonate, 10g of diatomaceous earth, and 150ml of dichloromethane. Slowly add the diluted solution A obtained above for about 30min. ...
Embodiment 2
[0027] Step 1: Preparation of Dibenzoyluridine (Formula IV)
[0028] Put 31.2g of protected fluororibose (formula I), 400ml of chloroform, and 23.1g of ditrimethylsilica-protected uracil (VI) in a 1000ml four-necked flask, stir, and the reaction solution will be heated to the range of 40-50°C, Slowly add 78.0 g of tin tetrachloride within 0.5h, after completion, heat to reflux (65°C), stir the reaction for 24h, sample HPLC to detect, the raw material basically reacted completely, and cool to room temperature (20°C) to obtain solution A.
[0029] In another 1000ml four-neck flask, add 80g of sodium bicarbonate, 20g of diatomaceous earth, and 200ml of chloroform, and slowly add the solution A obtained above, the reaction solution is cooled to about 10°C, 30ml of water is added to quench the reaction, and stirring is continued for 30 minutes. Heat up to reflux (65°C) and react for 30min. After completion, the reaction solution is cooled to 15°C and filtered. The filter cake is rinsed ...
Embodiment 3
[0034] Step 1: Preparation of Dibenzoyluridine (Formula IV)
[0035] Put 50g of protected fluororibose (formula I), 200ml of o-dichlorobenzene, and 33.8g of ditrimethylsilica-protected uracil (VI) into a 1000ml four-necked flask, stir, and the reaction solution will be heated to 50°C, at 0.5 Slowly add 125 g of tin tetrachloride within h. After completion, the temperature was raised to 75° C., and the reaction was stirred for 24 h. After sampling HPLC, the raw material was basically reacted completely, and the temperature was reduced to room temperature (20° C.). Add 300 ml of diluted solution A in dichloromethane.
[0036] In another 1000ml four-neck flask, add 130g of sodium bicarbonate, 30g of diatomaceous earth, and 500ml of dichloromethane, slowly add the solution A obtained above, cool the reaction solution to about 10°C, add 50ml of water to quench the reaction, and continue stirring 30min, heat to reflux (45℃) and react for 30min. After completion, the reaction solution is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com