Application of DLK1 gene in preparation of gastrointestinal stromal tumor diagnostic reagent
A gastrointestinal stromal tumor, gene technology, applied in the field of biomedicine, can solve the problems of increasing drug resistance, high price, waste of medical resources and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, gastrointestinal stromal tumor
[0059] The inventor first selected 12 cases of fresh surgically resected specimens of gastric GIST, and according to the currently used international GIST risk NIH grading standard (considering tumor size and mitotic phase), the specimens were numbered according to the increasing risk of tumor recurrence (low-risk 4 cases , 4 cases of medium risk, and 4 cases of high risk). The NimbleGen chip of Roche Company was used to study the gene expression profile microarray of the 12 samples, and the genes that were significantly up-regulated or down-regulated with the increase of tumor risk among different risk groups were screened out (p2).
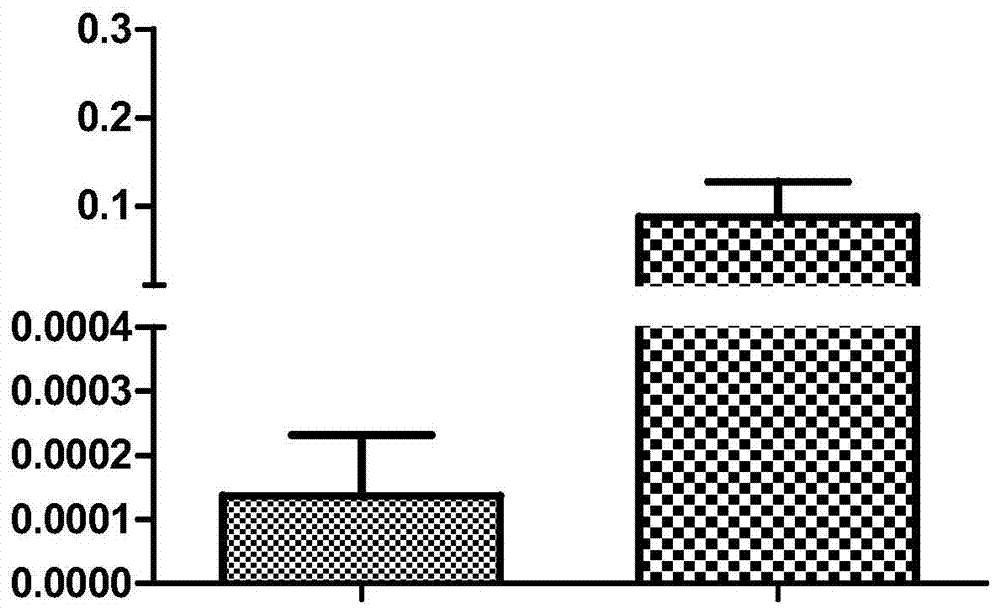
[0060] The results were screened to obtain a part of the genes whose expression occurred. It is particularly noteworthy that: in the expression profile microarray, the DLK1 gene was significantly up-regulated with the increase of tumor risk, p=0.00032, and the fold change of high risk vs. lo...
Embodiment 2
[0061] Example 2. Real-time quantitative PCR analysis of DLK1 gene expression in high-risk and low-risk patients with gastrointestinal stromal tumors
[0062] The genes screened above were verified by real-time quantitative PCR in another 24 GIST fresh tumor samples (14 high-risk cases and 10 low-risk cases).
[0063] 1.1 Main reagents
[0064] The RNA extraction reagent RNAiso Plus was purchased from Bao Biological Engineering Co., Ltd., the reverse transcription kit High Capacity cDNA Reverse Transcription Kits was purchased from Invitrogen, and the fluorescent quantitative PCR kit Power SYBR Green PCR Master Mix was purchased from Invitrogen.
[0065] The primer sequences of DLK1 and housekeeping gene β-actin are as follows:
[0066] DLK1: Upstream primer: CTTTCGGCCACAGCACCTAT (SEQ ID NO: 1); Downstream primer CCTCGCAGAATCCATTTTGGG (SEQ ID NO: 2).
[0067] β-actin: upstream primer: CTCCATCCTGGCCTCGCTGT (SEQ ID NO: 3); downstream primer GCTGTCACCTTCACCGTTCC (SEQ ID NO: 4)....
Embodiment 3
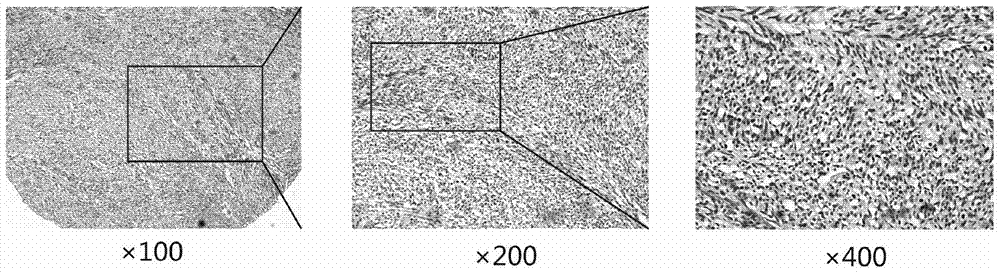
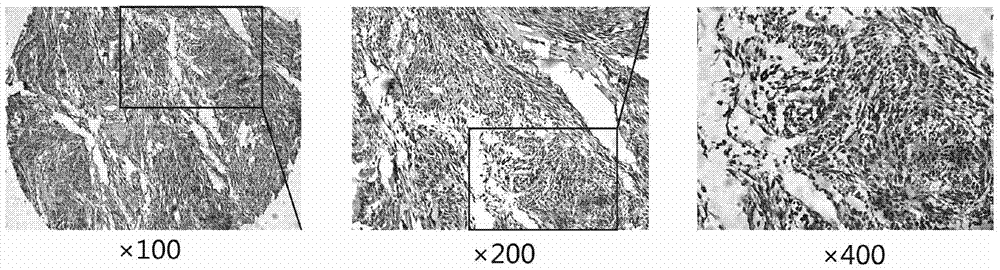
[0084] Example 3, the expression of DLK1 in the tissues of patients with gastrointestinal stromal tumors
[0085] 2.1 Main reagents
[0086] DLK1 antibody (ab21682) and horseradish peroxidase-labeled secondary antibody goat polyclonal anti-rabbit IgG (ab6721) were purchased from abcam. DAB chromogen and its substrate kit were purchased from Thermo Fisher. The rest of the reagents were of domestic analytical grade.
[0087] 2.2 Construction of gastrointestinal stromal tumor tissue chip array
[0088] Tumor tissues of patients with gastrointestinal stromal tumors were obtained from Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine. The construction of the chip array was completed by Suzhou Xinxin Biotechnology Co., Ltd., with a lattice diameter of 1.6mm and a layer thickness of 3mm. Chip 1 included tumor tissues of 139 patients with gastrointestinal stromal tumors and corresponding adjacent normal tissues, and chip 2 included tumor tissues of 189 p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com