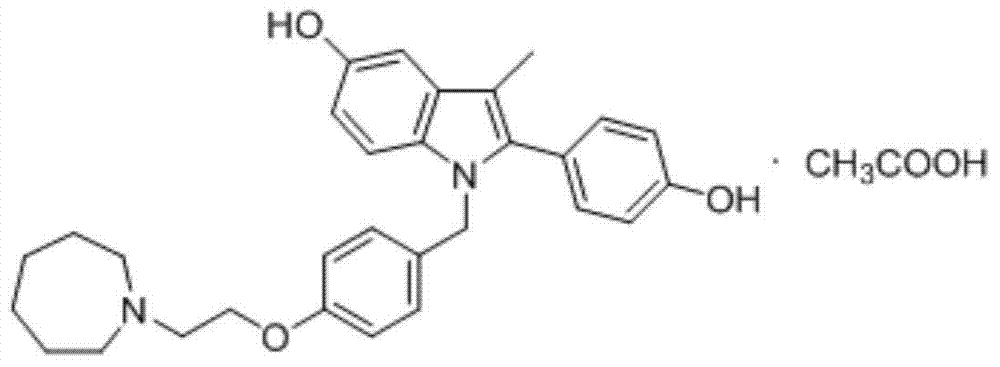
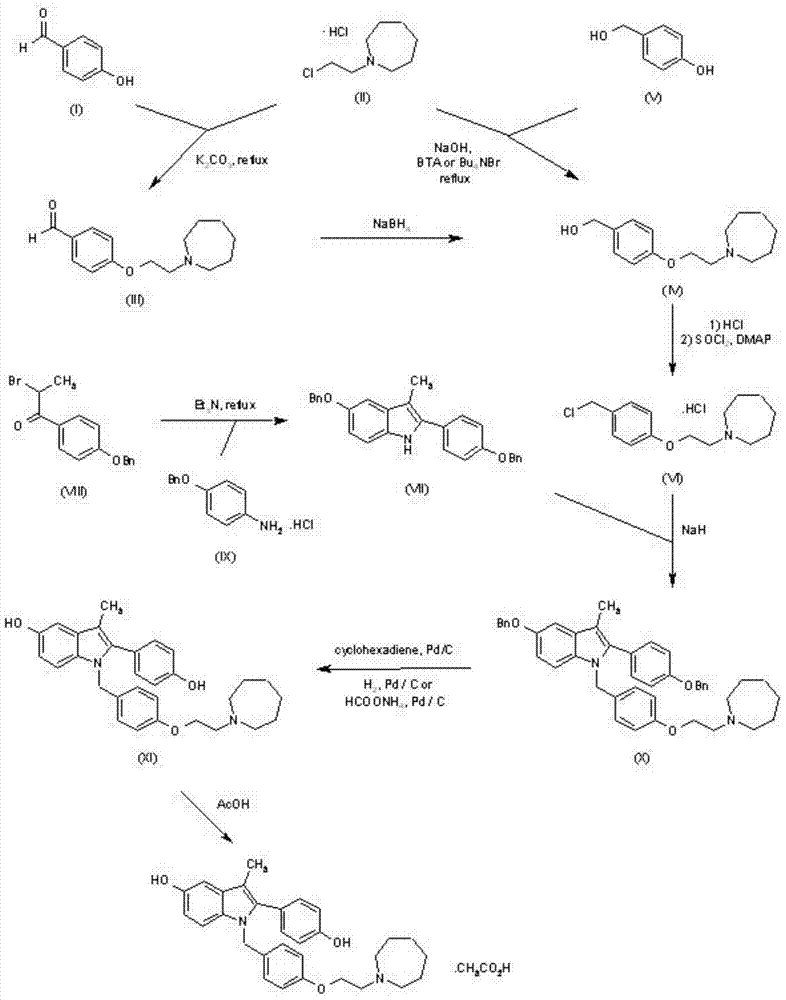
Preparation method of novel azacycloheptane derivative
A technology of cycloheptane base body and compound is applied in the field of pharmaceutical synthesis, which can solve the problems of high cost, many impurities and high yield, and achieves the effects of high toxicity, good product quality and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Preparation of 1,6-di-hexanediol-toluenesulfonate
[0018] Weigh 1.77 g of 1,6-hexanediol (15.0 mmol) and mix with 30 ml of anhydrous pyridine, stir and cool down to 0°C, and add 8.58 g of p-toluenesulfonyl chloride (45.0 mmol). After the addition, react at room temperature for 3 hours, add 3ml of water to quench the reaction, continue to stir for 30 minutes, add dichloromethane, add 1M hydrochloric acid dropwise under stirring to adjust the pH=6-7, stir for 30 minutes and then let it stand for stratification. The methyl chloride layer was washed with water and saturated brine successively, then dried (anhydrous sodium sulfate), filtered, dichloromethane was distilled off under reduced pressure, and the residue was first purified by column chromatography (dichloromethane / n-hexane / acetone=48:50 : 2), and then recrystallized (dichloromethane / n-hexane) to obtain 5.12 g of 1,6-dihexanediol di-p-toluenesulfonate (80%). Mp:77-79℃, 1 H NMR (500MHz, CDCl 3 )δ1.25-...
Embodiment 2
[0019] Example 2: Preparation of 2-(azepane)-1-ethanol
[0020] A certain amount of 1,6-dihexanediol di-p-toluenesulfonate is mixed with excess ethanolamine and toluene, stirred and heated to 120°C, and reacted for 2 hours. After the reaction is completed, excess ethanolamine is evaporated under reduced pressure, and di Chloromethane, then washed with water and saturated brine successively, dried (anhydrous magnesium sulfate), filtered, distilled to remove dichloromethane, the residue was purified by column chromatography (n-hexane / ethyl acetate=98:2) to obtain oil 2 -(azepane)-1-ethanol, yield 90%.
[0021] Bp: 97°C (14mmHg)
Embodiment 3
[0022] Example 3: Preparation of 1-(2-chloroethyl)azepane hydrochloride
[0023] Mix a certain amount of 2-(azepane)-1-ethanol with dichloromethane, add 5 molar equivalents of thionyl chloride and a catalytic amount of DMF, stir and heat to reflux, react for 2 hours, cool down and filter, filter The cake was washed with dichloromethane, recrystallized from isopropanol, and dried in vacuo to give 1-(2-chloroethyl)azepane hydrochloride in a yield of 85%. Mp: 208-209°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com