Camptothecin compound, and preparation method and application thereof
A compound, the technology of camptothecin, which is applied in the field of new camptothecin compounds, can solve the problems such as the research report on the pine wood nematode and the cinnabar spider mite test insect that have not been seen, and achieves good poisoning activity, high product purity, and synthetic simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
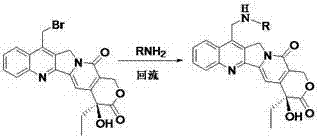
[0019] 7-( N Synthesis of -p-fluorophenyl-)-methylene-camptothecin (1)
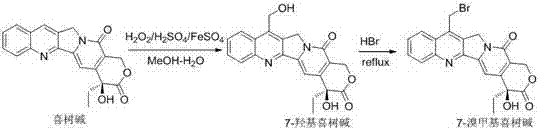
[0020] Synthesis of raw material 7-bromomethylcamptothecin : Dissolve 8.6mmol camptothecin in 90mL and 75mL water, add 75mL75% sulfuric acid dropwise, then add 8.6mmol ferrous sulfate heptahydrate. Under the condition of ice bath, 6.6 mmol 30% hydrogen peroxide was added dropwise. After dropping, stir at room temperature for 14 hours, add 40mml of water to dilute, a large amount of yellow solid is produced, filter, wash the solid with 10mml of water to obtain 2.7g of yellow solid (7-hydroxymethylcamptothecin). Dissolve 0.2mmol 7-hydroxymethylcamptothecin in a mixture of 10mL 45% hydrobromic acid and 0.35mL 97% concentrated sulfuric acid, heat to reflux for 6 hours, evaporate the solvent, and perform column chromatography (the elution system is chloroform: Methanol=40:1) Purified to obtain yellow solid 7-bromomethylcamptothecin. Synthetic method see literature method ( ChemMedChem. 2012, 7(12) ...
Embodiment 2
[0024] 7-( N Synthesis of -p-chlorophenyl-)-methylene-camptothecin (2)
[0025] The experimental procedure is the same as in Example 1, only p-chloroaniline is used instead of p-fluoroaniline.
[0026] The product detection data are as follows: Yield: 40%; Melting point: 243 °C (decomposition);1 H NMR (400MHz, DMS0-d 6 ) δ: 0.87-0.92 (t, J =7.2Hz, 3H, 19-H), 1.85-1.92 (m, 2H, 18-H), 5.12 (d, J =4.8Hz, 2H, -CH 2 -), 5.44 (s, 2H, 5-H), 5.52 (s, 2H, 17-H), 5.62 (s, 1H, NH), 6.53 (s, 1H, 20-OH), 7.37 (s, 1H , 14-H), 7.47 (d, J =8.8Hz, 2H, 2', 6'-H), 7.83-7.87 (m, 1H, 11-H), 7.91(d, J =8.8Hz, 2H, 3', 5'-H), 7.92 (t, 1H, J =7.2Hz, 10-H), 8.24 (d, J =8.4Hz, 1H, 12-H), 8.49 (d, J =8.4Hz, 1H, 9-H); MS-ESI m / z : 488.5[M+H] + .
Embodiment 3
[0028] 7-( N Synthesis of -p-bromophenyl-)-methylene-camptothecin (3)
[0029] The experimental procedure is the same as in Example 1, only p-bromoaniline is used instead of p-fluoroaniline.
[0030] The product detection data are as follows: Yield: 42%; Melting point: 257 °C (decomposition); 1 H NMR (400MHz, DMS0-d 6 ) δ: 0.89-0.93 (t, J =6.8Hz, 3H, 19-H), 1.86-1.91 (m, 2H, 18-H), 5.11(d, J =4.8Hz, 2H, -CH 2 -), 5.46 (s, 2H, 5-H), 5.56 (s, 2H, 17-H), 6.52 (s, 1H, 20-OH), 6.57 (s, 1H, NH), 7.42 (s, 1H , 14-H), 7.52 (d, J =8.8Hz, 2H, 2', 6'-H), 7.65 (m, 1H, 11-H), 7.81(d, J =8.8Hz, 2H, 3', 5'-H), 7.86 (t, 1H, J =7.2Hz, 10-H), 8.17 (d, J =8.4Hz, 1H, 12-H), 8.49 (d, J =8.4Hz, 1H, 9-H); MS-ESI m / z : 533.3[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com