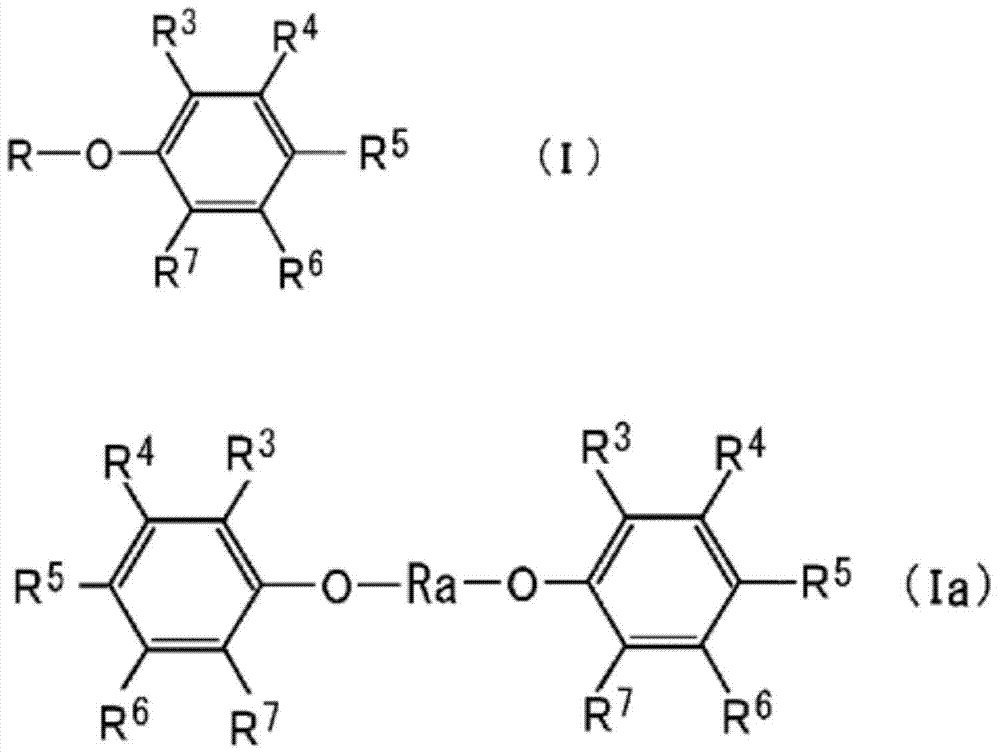
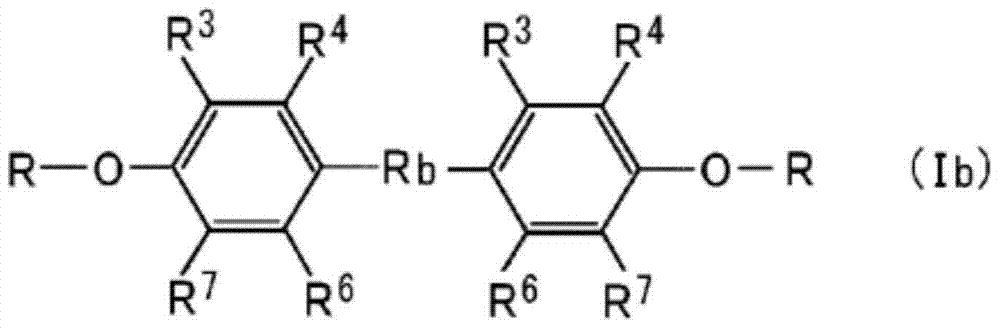
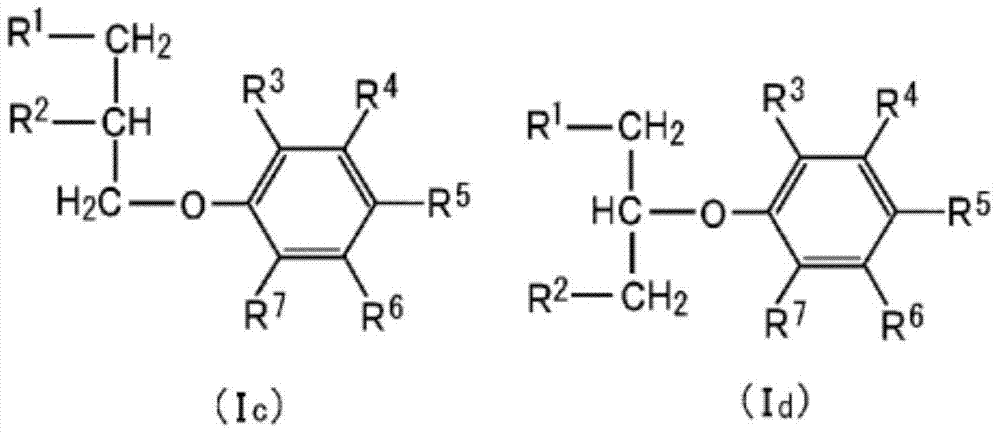
Propyl-phenyl-ether derivative and melanogenesis inhibitor, skin-lightening agent, antimicrobial agent, and cosmetic containing said propyl-phenyl-ether derivative
A technology of propyl phenyl ether and its derivatives, which can be applied in the direction of chemicals for biological control, biocides, skin care preparations, etc., and can solve the problems of not showing antibacterial effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0147] Hereinafter, specific embodiments of the present invention will be described using examples, but the scope of the present invention is not limited by the following examples.
Synthetic example 11-
[0148] Synthesis Example 11 - Synthesis of (4'-tert-butylphenoxy)propyl
[0149] Add sodium hydroxide (0.26g) and tetrabutylammonium bromide (0.43g) to 4-tert-butylphenol (1.00g), stir at room temperature, add bromopropyl (1.64g), and then After stirring for 5 hours, ethyl acetate and water were added, and the target substance was extracted with ethyl acetate. The extract was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and 1.37 g of the obtained residue was subjected to silica gel column chromatography. The mixture was eluted with hexane / ethyl acetate=20 / 1, and concentrated under reduced pressure to obtain a product. The obtained product was confirmed to have a different Rf value from each raw material and a spot of UV absorption by HPTLC analysis, and the raw material was identified as 1-(4'-tert-butylphenoxy)propyl group represented by the structural formula shown below.
[0150]
Synthetic example 21-
[0151] Synthesis Example 21 - Synthesis of (4'-tert-butylphenoxy)ethyl
[0152] Add sodium hydroxide (0.26g) and tetrabutylammonium bromide 0.43g to 4-tert-butylphenol (1.00g), stir at room temperature, add bromoethane (2.20g), and stir at 40°C After 5 hours, ethyl acetate and water were added, and the target substance was extracted with ethyl acetate. The extract was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and 0.97 g of the obtained residue was subjected to silica gel column chromatography. The mixture was eluted with hexane / ethyl acetate=20 / 1, and concentrated under reduced pressure to obtain a product. The obtained product was confirmed to be a compound having UV absorption by HPTLC analysis, and was identified as a 1-(4'-tert-butylphenoxy)ethyl group represented by the structural formula shown below from the raw material.
[0153]
[0154] Synthesis Example 31, Synthesis of 2-Di-Hydroxy-3-Phenoxypropyl
[0155] Add water (5.00g) and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com