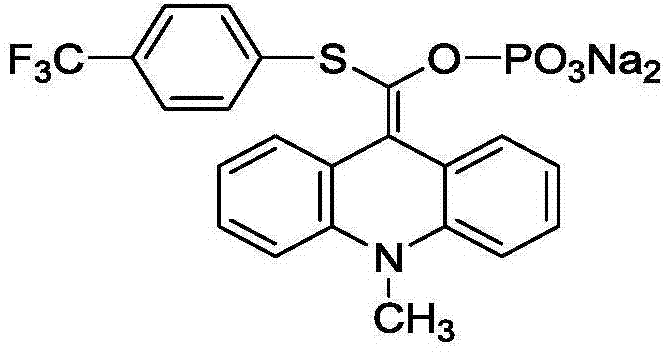
Acridinium ester derivative, synthesis method and application thereof
A technology of derivatives and acridinium esters, applied in the field of chemiluminescent acridinium ester derivatives and their synthesis, to achieve the effects of constant intensity, long luminescence duration and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
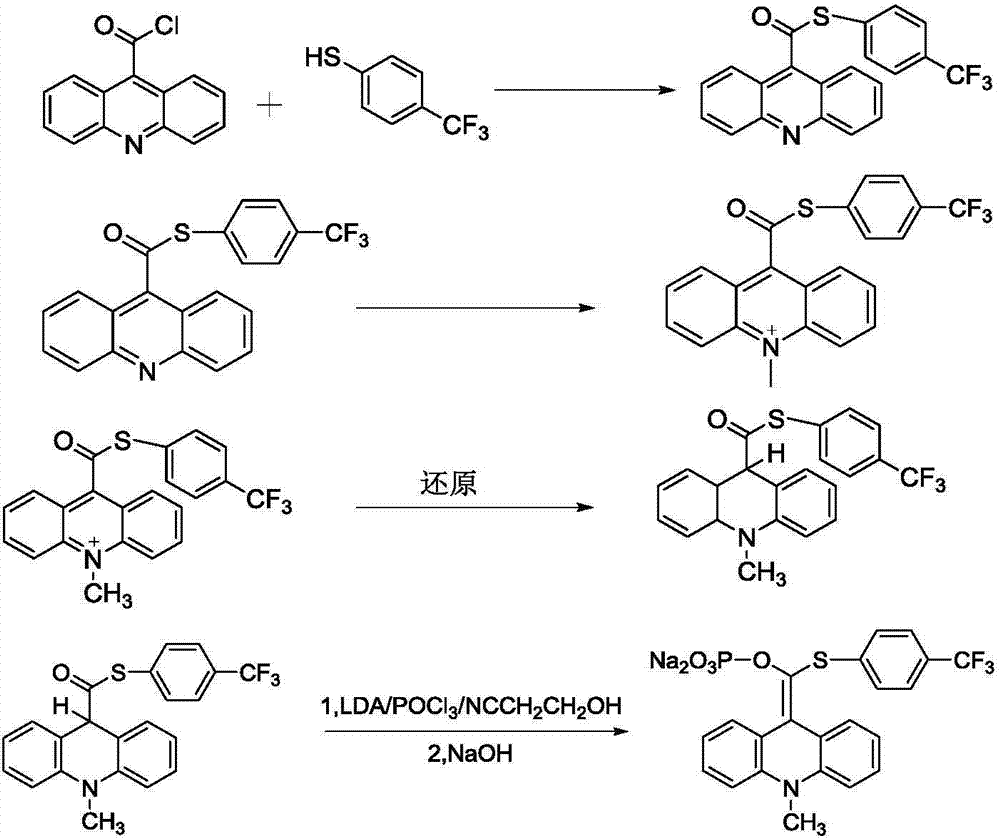
[0038] Example 1 Synthesis of 9-[(p-trifluoromethylphenylthio)-phosphoryloxymethylene]-10-methyl-9,10-dihydroacridine, disodium salt
[0039] 1. Synthesis of acridine-9-thiocarboxylic acid p-trifluoromethylphenyl ester
[0040] In a 250ml three-necked reaction flask, add 80ml of DCM (dichloromethane) as a solvent, add 1.78g of p-trifluoromethylthiophenol and 2.40g of acridinium-9-acid chloride, and add 2ml of triethylamine under stirring , and soon the reaction mixture turned orange, heated to reflux, stirred and reacted for about 2 hours. After the reaction was complete, the solvent was removed, and the obtained solid was washed with 5% aqueous sodium bicarbonate and deionized water, filtered, and the filter cake was dried to obtain 3.0 g of a brownish-yellow solid product with a yield of 78.3%.
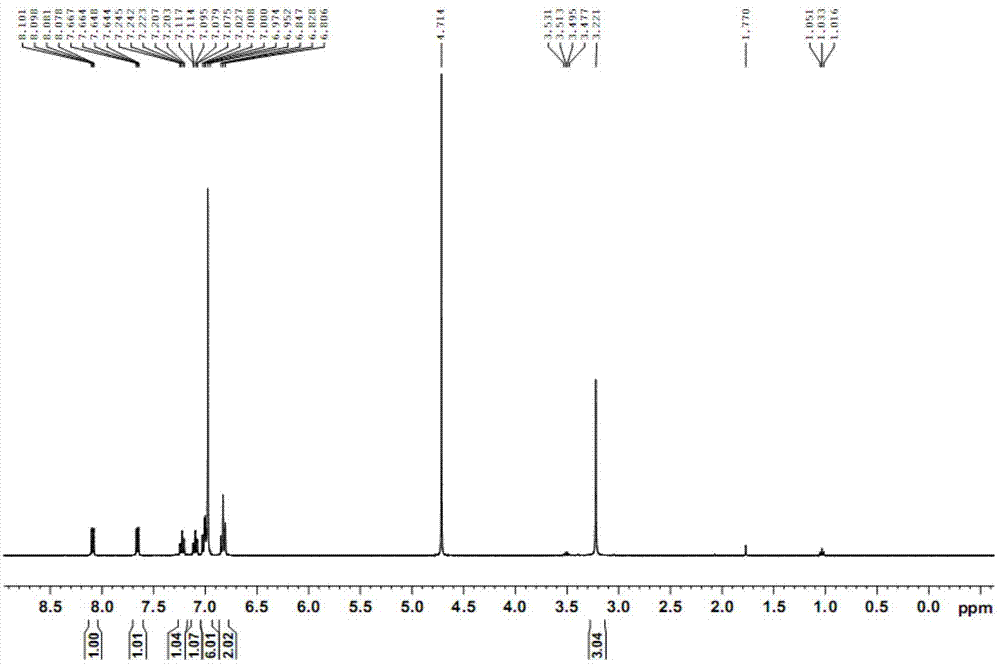
[0041] 1 H NMR (CDCl 3 ) δ7.47~7.50(m, 2H), 7.60~7.65(m, 4H), 7.82~7.85(m, 2H), 8.12(d, 2H), 8.30(d, 2H).
[0042] 2. Synthesis of 10-methyl-acridine-9-thiocarboxylic acid p-tri...
experiment example 1
[0051] Experimental example 1 9-[(p-trifluoromethylphenylthio)-phosphoroxymethylene]-10-methyl-9,10-dihydroacridine, application experiment of disodium salt
[0052] 1. 9-[(p-trifluoromethylphenylthio)-phosphoryloxymethylene]-10-methyl-9,10-dihydroacridine, the chemiluminescent substrate working solution of disodium salt prepare
[0053] experiment material:
[0054]
[0055] Preparation method:
[0056] Add 700ml of deionized water into the light-proof container, add 24.0g of TRIS, 0.03g of cetyltrimethylammonium chloride, 9.0g of sodium chloride, and 5.0mg of lucigenin in sequence. Hydrochloric acid to adjust the pH to 9.35±0.1. Add 9-[(p-trifluoromethylphenylthio)-phosphoryloxymethylene]-10-methyl-9,10-dihydroacridine, 0.2g of disodium salt, stir to dissolve and use deionized Make up to 1 liter of water. Verify that the pH is within the range of 9.35±0.1, filter through a 0.22 micron pore size filter, and store at 2-8°C.
[0057] 2. Performance detection of lumines...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com