Polymerizable thioxanthone carbazole visible light initiator containing co-initiator amine and preparation method thereof
A technology based on thioxanthone carbazoles and visible light, applied in organic chemistry and other fields, can solve problems such as poor compatibility, easy migration of fragments, and low initiation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The product of the present invention 3-(diethylamino)-2-(13-oxothiochromeno[2,3-B]carbazol-7-(13H)-yl)propyl methacrylate, which The structural formula is:
[0051]
[0052] Preparation of product 3-(diethylamino)-2-(13-oxothiochromeno[2,3-B]carbazol-7-(13H)-yl)propyl methacrylate of the present invention method, the detailed steps are as follows:
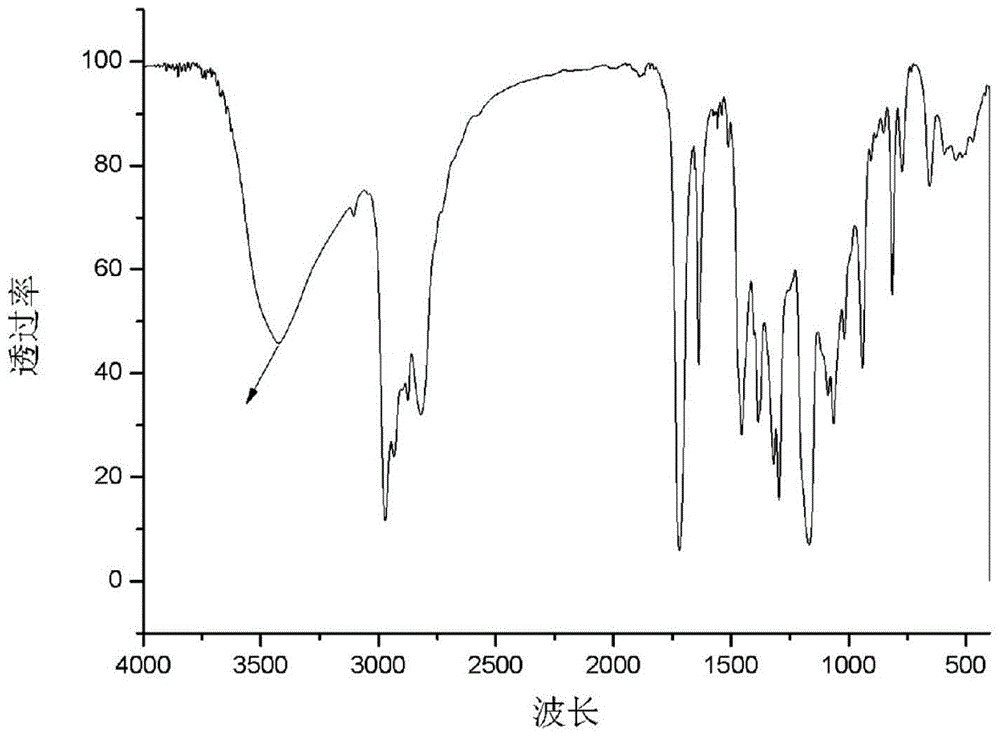
[0053] a. Put 29.24g (0.4mol) of diethylamine into a 100ml reaction flask, start stirring, heat to 56°C, slowly add 28.40g (0.2mol) of glycidyl methacrylate (GMA) into the reaction flask, and react 2h; warming up to 80°C, reacting for 3h; after the reaction, the resulting reactant was distilled to remove residual diethylamine to obtain 42.80g 3-(diethylamino)-2-hydroxypropyl methacrylate ( GMA-DEA) (see the attached infrared spectrum figure 1 );
[0054] b. Add 4.30g (0.02mol) of GMA-DEA and 0.79g (0.01mol) of pyridine into a 100ml reaction flask containing 30ml (44.50g) of chloroform at 1 to 3°C, and then dropwise ad...
Embodiment 2
[0058] Embodiment 2: basically the same as Embodiment 1, the difference is:
[0059] Preparation of product 3-(diethylamino)-2-(13-oxothiochromeno[2,3-B]carbazol-7-(13H)-yl)propyl methacrylate of the present invention method, the detailed steps are as follows:
[0060] a. Put 29.24g (0.4mol) of diethylamine into a 100ml reaction bottle, start stirring, heat to 40°C, slowly add 28.40g (0.2mol) of GMA into the reaction bottle, react for 1h; raise the temperature to 70°C for 4h ; After the reaction finished, the gained reactant was distilled to remove remaining diethylamine to obtain 42.08g GMA-DEA;
[0061] b. Add 4.30g (0.02mol) GMA-DEA into a 100ml reaction flask containing 40ml (59.20g) chloroform at 1-3°C, then add 5.40g (0.02mol) PBr dropwise 3 and a mixed solution of 10ml (14.80g) chloroform, reacted at 1-3°C for 1h; raised the temperature to 70°C for 4h;
[0062]After the reaction finished, the gained reactant was cooled to 20°C, added in 15ml of distilled water, then ...
Embodiment 3
[0065] Embodiment 3: basically the same as Embodiment 1, the difference is:
[0066] Preparation of product 3-(diethylamino)-2-(13-oxothiochromeno[2,3-B]carbazol-7-(13H)-yl)propyl methacrylate of the present invention method, the detailed steps are as follows:
[0067] a. Put 14.63g (0.2mol) of diethylamine into a 100ml reaction flask, start stirring, and heat to 60°C; slowly add 28.40g (0.2mol) of GMA into the reaction flask at this temperature, and react for 4 hours; heat up to Reaction at 70°C for 3 hours; after the reaction, the obtained reactant was distilled to remove residual diethylamine to obtain 38.95 g of GMA-DEA;
[0068] b. Add 4.30g (0.02mol) GMA-DEA and 1.58g (0.02mol) pyridine into a 100ml reaction flask containing 50ml (74.00g) chloroform at 1-3°C, then add 16.20g (0.06 mol)PBr 3 and a mixed solution of 15ml (22.20g) of chloroform, reacted at 1-3°C for 2h; raised the temperature to 40°C for 5h;
[0069] After the reaction was finished, the gained reactant ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com