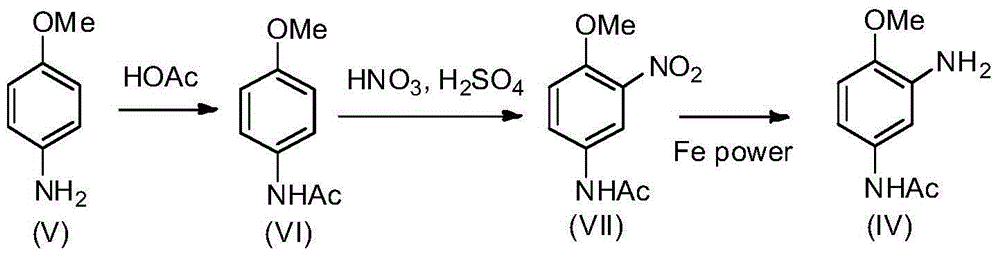
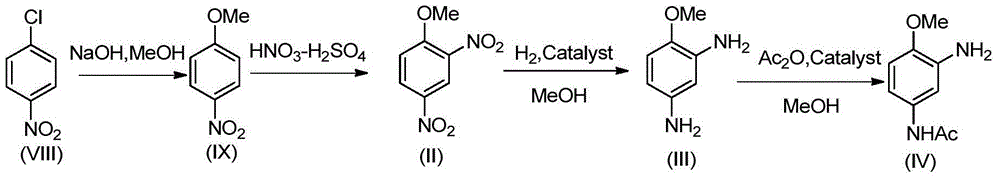
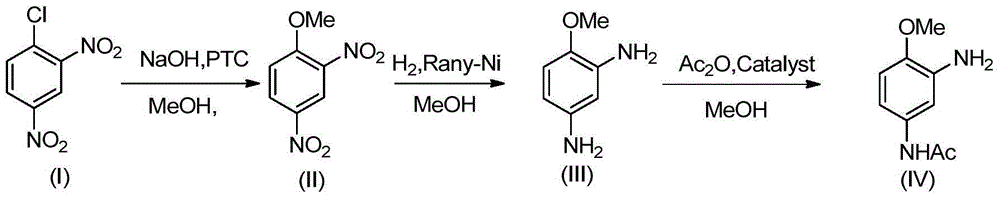
Synthesis process of 2-amino-4-acetamino anisole
A technology for acetamidoanisole and diaminoanisole, which is applied in the field of preparation of organic compounds, can solve the problems of unstable yield and quality of target compounds, unsuitable for industrialized production, low industrial safety factor, etc., and achieves reduction of side effects. The probability of product formation, the reduction of dosage, the effect of high industrial safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Weigh 1000g of 2,4-dinitrochlorobenzene (I), 1500g of methanol into a four-neck flask, 25g of PEG4000, heat up to 60°C, stir; add 198g of sodium hydroxide in batches within 2.5 to 3.0 hours, hydrogen Add sodium oxide, keep warm, and the reaction ends. Methanol was recovered by distillation and washed with water to obtain 950.8 g (99%) of 2,4-dinitroanisole (II), with a yield of 96.9%.
[0052] The NMR data of 2,4-dinitroanisole (II) are as follows: 1 HNMR (500MHz, CDCl 3 ): δ8.83(d,1H), 8.4~8.5(dd,1H), 8.72(d,1H), 3.78(s,3H).
Embodiment 2
[0054] Put 950.8g (99%) 2,4-dinitroanisole (II), 3803.2g methanol and 0.95g Raney-Ni catalyst, the system pH=8, the temperature was raised to 70°C, and 1.0MPa hydrogen gas was continuously introduced, The reaction is complete. The catalyst Raney-Ni was recovered by filtration. The HPLC purity of the active ingredient 2,4-diaminoanisole (III) in the mother liquor was 99.3%, and the conversion rate was 100%. The mother liquor was directly used for the acylation reaction without treatment.
[0055] Add 189.6g of ammonium bicarbonate to the above mother liquor, cool to -5°C, add 489.7g of acetic anhydride, after the addition is complete, raise the temperature to 5°C and keep warm until the reaction is complete. Distill 3042g of methanol, add water to the residue, cool and crystallize, and suction filter to obtain 770.2g (HPLC purity 99.5%) 2-amino-4-acetamidoanisole (IV), 2,4-diacetamidophenyl The amount of methyl ether generated is below 1% (measured by HPLC), and the total yiel...
Embodiment 3
[0058] Weigh 1000g of 2,4-dinitrochlorobenzene (I), 2500g of methanol into a four-neck flask, 15g of benzyltriethylammonium chloride, heat up to 70°C, and stir; within 2.5 to 3.0 hours, divide Add 196g of sodium hydroxide in batches, add sodium hydroxide, keep warm, and the reaction ends. Methanol was recovered by distillation and washed with water to obtain 928.8 g (HPLC purity 99.2%) of 2,4-dinitroanisole (II), with a yield of 94.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com