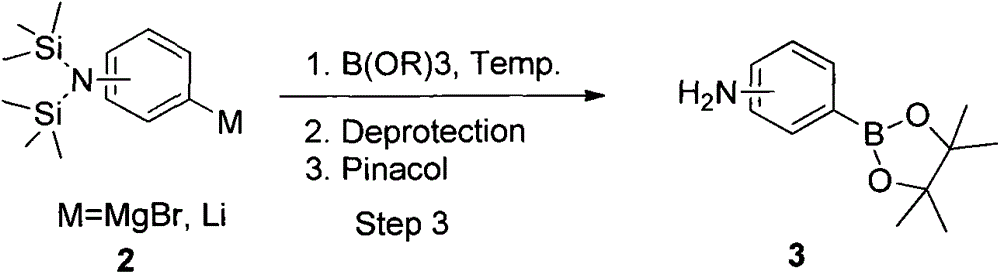
A method of synthesizing aminophenylboronic acid pinacol ester
A technology for synthesizing aminophenylboronic acid and aminobromobenzene, which is applied in the field of boron chemical synthesis and can solve problems such as difficult determination of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
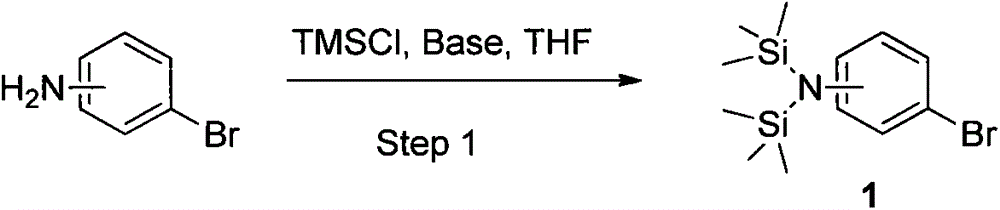
[0023] Example 1: Synthesis of 2-bis(trimethylsilyl)aminobromobenzene (1, ortho):
[0024] Under argon protection, in a 3L three-neck flask equipped with magnetic stirring, 2-aminobromobenzene (172 g, 1.0 mol) and triethylamine (303.6 g, 3.0 mol) were added to 850 ml of anhydrous THF solvent, and the After stirring, the reaction solution was cooled to about 0°C, and then 3.0 equivalents of trimethylchlorosilane (325.9 g, 3 moles) was slowly added dropwise, and the temperature of the system rose to 30°C during the dropwise addition. Stir for 10 minutes after dropping, and then react at 40-60°C for 1-3 hours. TLC detects that the reaction is complete. The developing solvent is: n-hexane / ethyl acetate=10:1. At this time, it is the intermediate protected by the previous trimethylsilyl group.
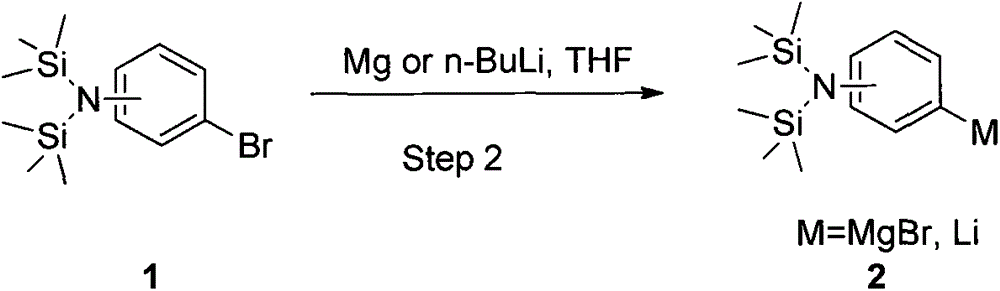
[0025] Cool down to -10-0°C, and maintain the temperature by adding 1.5 equivalents of commercially available 3M methylmagnesium chloride tetrahydrofuran solution (500 ml, 1.5 moles), follow...
Embodiment 2
[0026] Example 2: Synthesis of 3-bis(trimethylsilyl)aminobromobenzene (1, meta):
[0027] Under nitrogen protection, in a 3L three-neck flask equipped with magnetic stirring, 3-aminobromobenzene (172 g, 1.0 mol) and diisopropylethylamine (387.7 g, 3.0 mol) were added to 850 ml of anhydrous THF solvent , start stirring, and cool the reaction liquid to about 0°C, then slowly add 3.0 equivalents of trimethylchlorosilane (325.9 g, 3 moles) dropwise, and the temperature of the system rises to 30°C during the dropwise addition. Stir for 10 minutes after dropping, and then react at 40-60°C for 1-3 hours. TLC detects that the reaction is complete. The developing solvent is: n-hexane / ethyl acetate=10:1. At this time, it is the intermediate protected by the previous trimethylsilyl group.
[0028] Cool down to -10-0°C, and maintain the temperature by adding 1.5 equivalents of a commercially available 3.2M methylmagnesium bromide 2-methyltetrahydrofuran solution (469 ml, 1.5 moles), and t...
Embodiment 3
[0029] Example 3: Synthesis of 4-bis(trimethylsilyl)aminobromobenzene (1, p-position):
[0030] Under nitrogen protection, add 4-aminobromobenzene (172 g, 1.0 mol) and triethylamine (303.6 g, 3.0 mol) into 850 ml of anhydrous THF solvent in a 3L three-necked flask equipped with magnetic stirring, and start stirring , the reaction solution was cooled to about 0°C, and then 3.0 equivalents of trimethylchlorosilane (325.9 g, 3 moles) was slowly added dropwise, and the system temperature rose to 30°C during the dropwise addition. Stir for 10 minutes after dropping, and then react at 40-60°C for 1-3 hours. TLC detects that the reaction is complete. The developing solvent is: n-hexane / ethyl acetate=10:1. At this time, it is the intermediate protected by the previous trimethylsilyl group.
[0031] Cool down to -10-0°C, and maintain the temperature by adding 1.5 equivalents of a commercially available 2M isopropylmagnesium chloride tetrahydrofuran solution (750 ml, 1.5 moles), followe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com