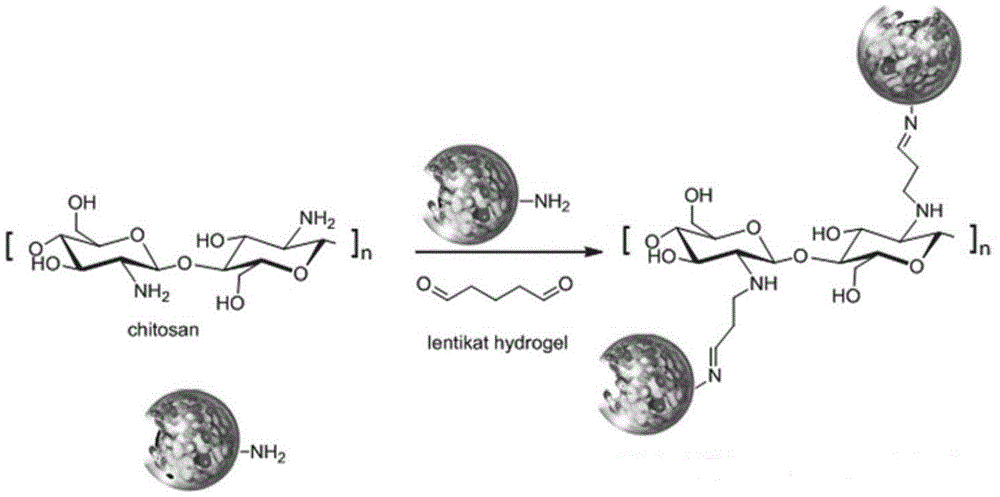
Immobilized transaminase and applications thereof in synthesizing of Sitagliptin intermediate
A technology of immobilized transaminase and transaminase, which is applied in the application field of immobilized transaminase and asymmetric synthesis of sitagliptin intermediates, can solve the problems of difficult separation, difficult mass transfer, and large loss of enzyme activity, and achieve simple separation, The effect of strong binding and less loss of enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 Preparation of recombinant transaminase expression transformant
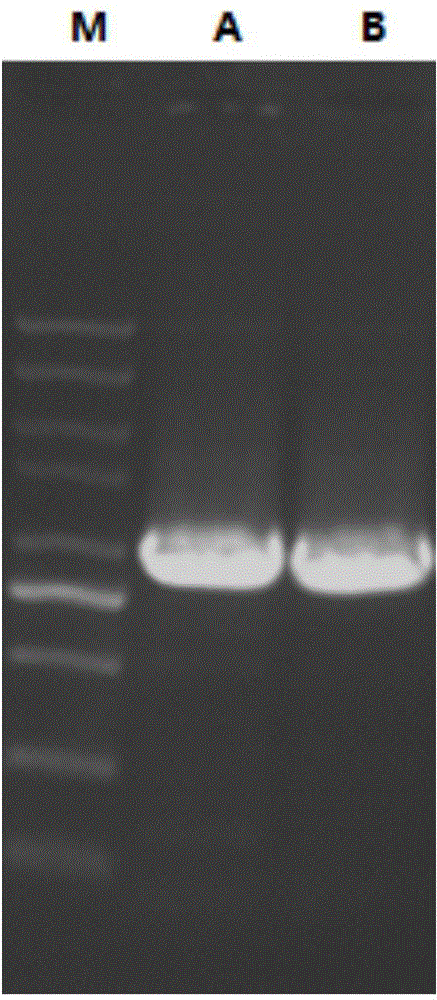
[0051] According to Chinese Patent Application No. 201410169882.4, the recombinant expression vector pET21a-MvAT containing SEQ ID No.1 was constructed, digested with restriction endonucleases Nde Ⅰ and EcoR Ⅰ at 37°C for 8 hours, purified by agarose gel electrophoresis, and agarose Glycogel DNA Recovery Kit recovers target fragments. Under the action of T4 DNA ligase, the target fragment was ligated with the plasmid pET28a digested with Nde Ⅰ and EcoR Ⅰ at 16°C overnight to obtain the recombinant expression plasmid pET28a-MvAT. The recombinant expression plasmid was transformed into Escherichia coli (E.coli) DH5α competent cells, the transformation conditions were 45°C, heat shock for 90 seconds, and the positive recombinants were screened on the resistance plate containing kanamycin, Pick a single clone and verify the positive clone by colony PCR (see figure 2 A). Cultivate the recombin...
Embodiment 2
[0052] Expression of embodiment 2 recombinant transaminases
[0053] Inoculate the recombinant E.coli BL21 (DE3) obtained in Example 3 into LB medium containing kanamycin (peptone 10g / L, yeast extract 5g / L, NaCl 10g / L, pH 7.0), shake at 37°C Cultivate overnight, insert in a 500ml Erlenmeyer flask equipped with 100ml LB medium according to the inoculum size of 1% (v / v), put 37 ℃, 180rpm shaker shaking culture, when the OD of culture solution 600 When it reached 0.6, IPTG with a final concentration of 0.2 mmol / L was added as an inducer, and after induction at 35°C for 12 hours, the culture medium was centrifuged to collect cells and washed twice with saline to obtain resting cells.
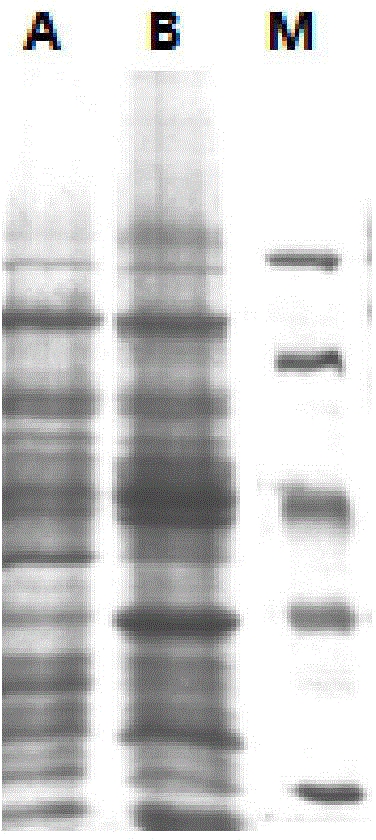
[0054] 1 g of the obtained resting cells was resuspended in 5 mL of water, homogenized under high pressure, and the cells were broken to obtain a crude enzyme solution. The protein content of crude enzyme solution measured by Bradford method was 400mg / g. The crude enzyme solution was analyzed by p...
Embodiment 3
[0055] The preparation of embodiment 3 immobilized enzyme
[0056] Mix 1.5% (w / v) sodium alginate with the crude enzyme solution obtained in Example 2, and melt it in a water bath at 37°C; after mixing well, add 1% (v / v) glutaraldehyde, and put it in a shaker 30min, and stood in a refrigerator at 4°C for 3h to obtain a mixed solution. Then the mixed solution was injected dropwise into 2% (w / v) calcium chloride solution to form gel beads; filtered and washed twice with 0.9% (w / v) sodium chloride solution to obtain the immobilized enzyme .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com