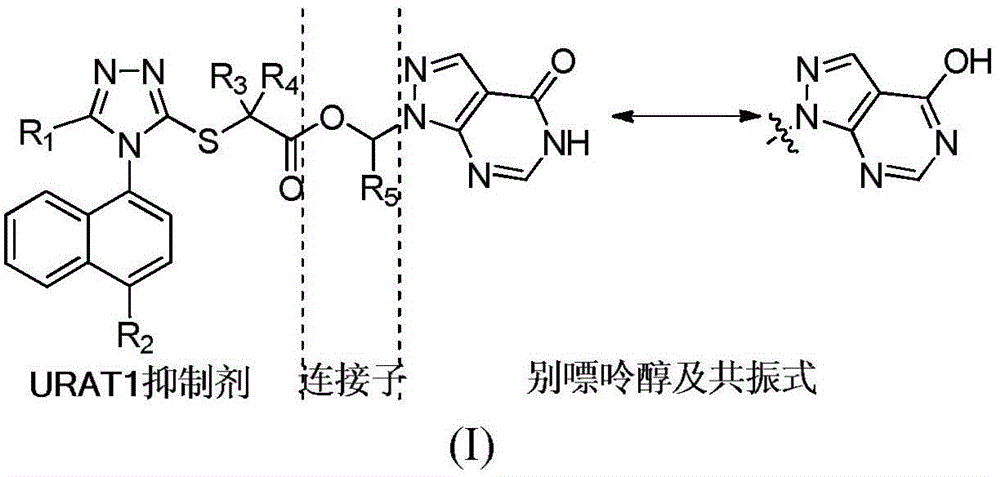
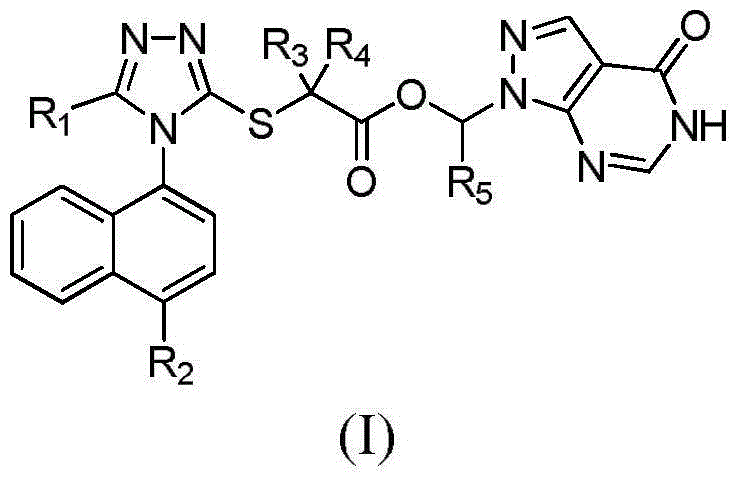
Compound with effect of treating and preventing hyperuricemia or gout and preparation method as well as application thereof
A technology for hyperuricemia and compounds, applied in the field of medicine, can solve problems such as lowering, insensitivity to probenecid, and inability to reach serum uric acid levels, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
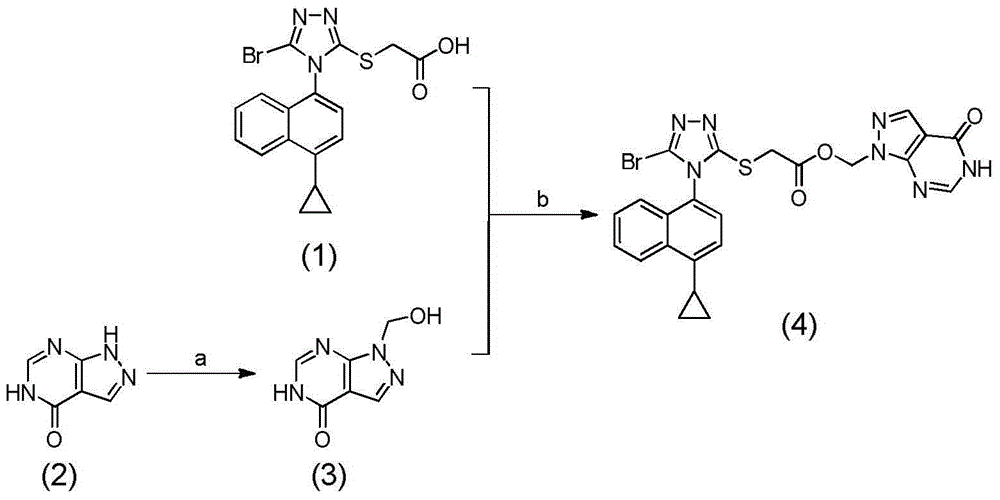
[0049] Embodiment 1: the preparation of the compound of No. 1 in table 1
[0050] (4-oxo-4,5-dihydro-1-hydrogen-pyrazolo[3,4-d]pyrimidin-1-yl)methyl-2-((5-bromo-4-(4-ring Preparation of methyl propylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate (4, compound no. 1 in Table 1):
[0051]
[0052] a) Preparation of 1-(hydroxymethyl)-1H-pyrazolo[3,4-d]pyrimidin-4(5H)-one (3):
[0053] Take a 250mL single-necked bottle and dry it, add the raw material allopurinol (5.0g, 36.7mmol) into it, and add 250mL of a 2M formaldehyde solution with a pH of 7 that has been prepared. Stir overnight at room temperature for 24 hours, a white solid can be seen to be precipitated, and filtered to obtain 1-(hydroxymethyl)-1H-pyrazolo[3,4-d]pyrimidin-4(5H)-one 3, 6.0 g, yield 98% , MS (ESI) m / z: 166.03 (M+H + ), used directly in the next reaction.
[0054] b) (4-oxo-4,5-dihydro-1-hydrogen-pyrazolo[3,4-d]pyrimidin-1-yl)methyl-2-((5-bromo-4-(4 Preparation of -cyclopropylnaphthalen-1-yl)-4H-...
Embodiment 2
[0056] Embodiment 2: the preparation of the compound of No. 2 in table 1
[0057] (4-oxo-4,5-dihydro-1-hydrogen-pyrazolo[3,4-d]pyrimidin-1-yl)methyl-2-((5-bromo-4-(4-ring Preparation of methyl propylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)-2-methylpropanoate (5, compound no. 2 in Table 1):
[0058]
[0059] a) 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylsulfanyl)-2-methylpropionic acid ethyl ester (2) Preparation:
[0060] Take a 25mL round-bottomed flask for drying, and the raw material 5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-l,2,4-triazole-3-thiol (0.200g, 0.578mmol ), ethyl 2-bromo-2-methylpropionate (0.112g, 0.578mmol) and diisopropylethylamine (0.224g, 1.734mmol) were dissolved in DMF (5mL), heated to 60°C and stirred for reaction one day. After the reaction was completed, the solvent was evaporated to dryness under reduced pressure, dissolved in ether, washed and extracted with 1M hydrochloric acid solution, the aqueous layer was extra...
Embodiment 3
[0065] Embodiment 3: the preparation of the compound of No. 3 in table 1
[0066] Compound No. 3 in Table 1 (4-oxo-4,5-dihydro-1-hydrogen-pyrazolo[3,4-d]pyrimidin-1-yl)methyl-2-((4-( 4-cyclopropylnaphthalene-1-yl)-4H-1,2,4-triazol-3-yl)thio)methyl acetate can be corresponding raw material 2-((4-(4-cyclopropylnaphthalene -1-yl)-4H-1,2,4-triazol-3-yl)thio)acetic acid was prepared by referring to the general method of the compound in Example 1. Yield 78%, MS (ESI) m / z: 474.13 (M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com