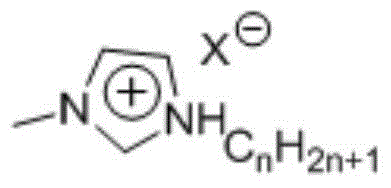
Method of synthesizing 5-hydroxymethyl furfural
A technology of hydroxymethyl furfural and ionic liquid, which is applied in the field of synthesizing 5-hydroxymethyl furfural, can solve the problems of unsatisfactory reaction effect and high viscosity of ionic liquid, and achieves improved catalytic reaction system, low volatility and low toxicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The following examples will further illustrate the present invention, but do not limit the present invention thereby.
[0030] For this reaction in the literature or previous patents, low-content glucose raw materials (glucose accounts for 5-20wt% of the mass of the ionic liquid) are often used for reaction tests in order to obtain higher selectivity. However, in production, in order to improve the utilization rate of the reaction bed, it is necessary to use high-concentration raw materials as much as possible. In this example, glucose accounts for more than 30wt% of the mass of the ionic liquid as a raw material, and a series of comparative experiments were done with reference to the reaction conditions (temperature, ionic liquid, catalyst) in the literature to illustrate the implementation of the present invention and the resulting effects .
[0031] The commonly used reaction steps are as follows: Weigh a certain amount of [BMIM]Cl, D-glucose and CrCl 3 ·6H 2 O is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com