Silver nanorod and preparing method thereof
A technology of silver nanorods and silver nitrate, applied in the field of nanomaterials, can solve the problems of many influencing factors, many by-products, low yield and the like, and achieves the effects of high preparation, easy operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The method for preparing silver nanorods in this embodiment includes the following steps:
[0038] Step 1: Take 3.5g polyvinylpyrrolidone (MW≈55000) and dissolve it in 35mL ethylene glycol (AR) (0.1g / mL), add it to the flask after dissolving it, magnetically stir and heat. Keep the temperature for 15 minutes after the temperature reaches 160℃;
[0039] Step 2: Add 80μL of 0.2mol / L sodium chloride glycol solution into the flask;
[0040] Step 3: After 5 minutes, add 4mL of 1mol / L silver nitrate ethylene glycol solution to the flask at a rate of 5% / min of the total volume. After adding 1 / 2, the remaining silver nitrate ethylene glycol solution is used for one time Added to the flask;
[0041] Step 4: The mixture is kept at constant temperature for 2 hours under magnetic stirring and condensing reflux; after it is naturally cooled, it is washed and centrifuged to obtain silver nanorods.
[0042] The yield of silver nanorods in this example was 85%.
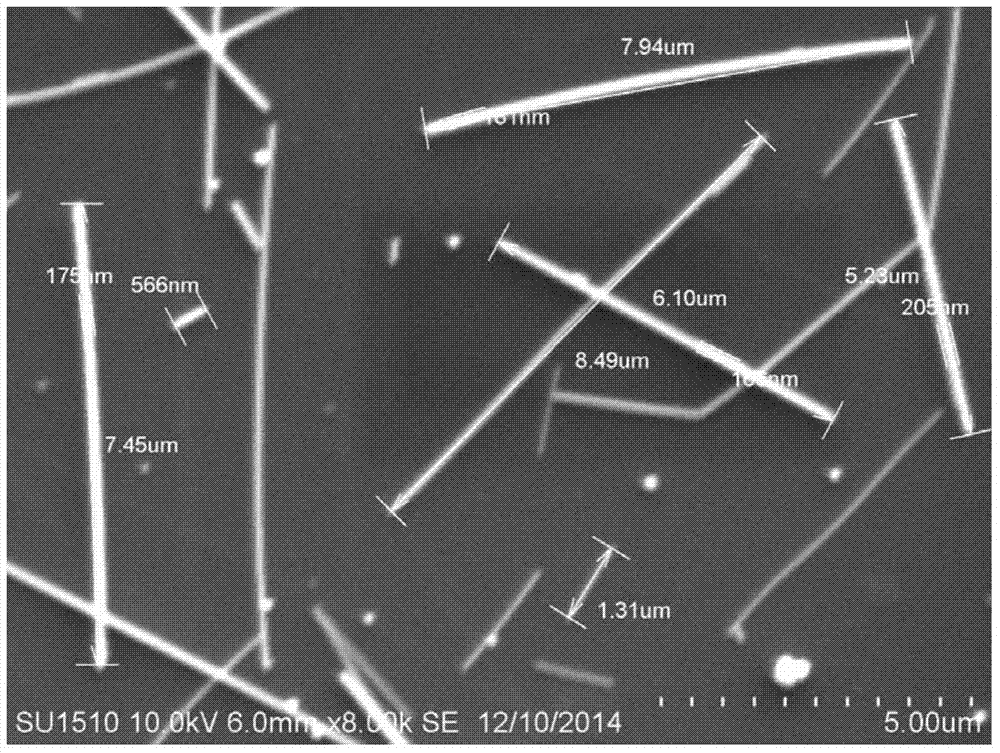
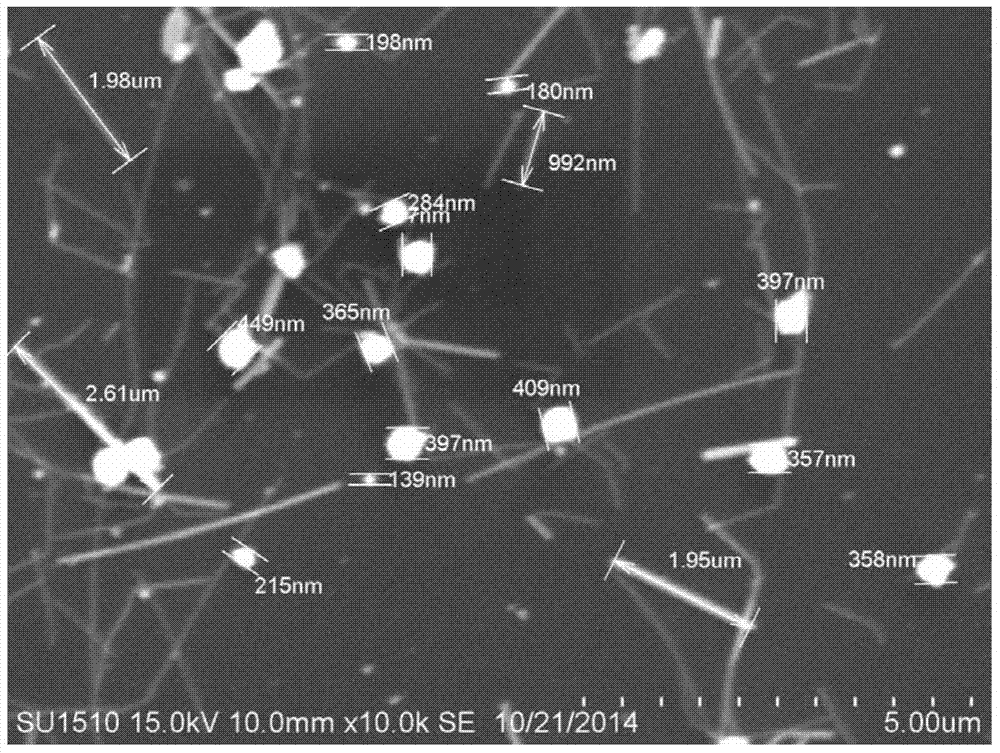
[0043] Characterize the prepare...
Embodiment 2
[0045] The method for preparing silver nanorods in this embodiment includes the following steps:
[0046] Step 1: Dissolve 3.5g polyvinylpyrrolidone (MW≈55000) in 35mL glycerol (AR), add it to the flask after it is completely dissolved, stir magnetically and heat. Keep the temperature for 15 minutes after the temperature reaches 160℃;
[0047] Step 2: Add 60μL 0.2mol / L ferric chloride and glycerin solution into the flask;
[0048] Step 3: After 5 minutes, add 4mL of 1mol / L silver nitrate glycerol solution into the flask at a rate of 5% / min of the total volume, and then drop the remaining silver nitrate glycerol solution at once. Added to the flask;
[0049] Step 4: The mixed solution is kept at constant temperature for 2 hours under magnetic stirring and condensing reflux; after it is naturally cooled, it is washed and centrifuged to obtain silver nanorods.
[0050] The yield of silver nanorods in this example was 80%.
[0051] The electron micrograph of the silver nanorods of this exa...
Embodiment 3
[0053] The method for preparing silver nanorods in this embodiment includes the following steps:
[0054] Step 1: Take 3.5g polyvinylpyrrolidone (MW≈55000) and dissolve it in 35mL ethylene glycol (AR), add it to the flask after it is completely dissolved, stir and heat it magnetically. Keep the temperature for 15 minutes after the temperature reaches 160℃;
[0055] Step 2: Add 80μL of 0.2mol / L potassium bromide glycol solution into the flask;
[0056] Step 3: After 5 minutes, add 4mL of 1mol / L silver nitrate ethylene glycol solution to the flask at a rate of 5% / min of the total volume. After adding 1 / 2, the remaining silver nitrate ethylene glycol solution is used for one time Added to the flask;
[0057] Step 4: The mixture is kept at a constant temperature for 2 hours under magnetic stirring and reflux. After natural cooling, washing and centrifugation to obtain silver nanorods.
[0058] The yield of silver nanorods in this example was 83%.
[0059] The electron micrograph of the si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com