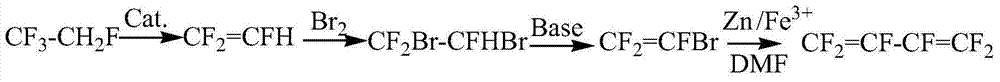Method for synthesizing hexafluoro-1,3-butadiene
A technology of butadiene and trifluoroethylene, which is applied in the field of synthesizing hexafluoro-1,3-butadiene, can solve the problems of restricting the industrial production of hexafluorobutadiene, and achieve safe synthesis process, high yield, and source convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Load 20ml of Cr-Mg catalyst into a fixed bed reactor. Under the protection of 50ml of nitrogen, the catalyst was first dried at 10°C / min to 200°C for 2 hours, then raised to 400°C at a rate of 10°C / min and dried for 2 hours, then cooled to 330°C, and then passed through The catalyst was activated with 50 ml / min of hydrogen fluoride (HF) gas for 2 hours. This completes the catalyst activation process.
[0037] Heat the reactor to 350°C, then, 50ml / min tetrafluoroethane and 10ml / min nitrogen enter the mixing chamber together and mix well. Afterwards, through the reactor until the buffer bottle, water washing bottle, concentrated alkali solution, and liquid bromine absorption pool, after the experiment, the product is mainly distributed in the cooling collector. The collected product was subjected to GC analysis. GC results showed that the product contained 95.0% 1,2-dibromo-1,1,2-trifluoroethane. Since trifluoroethylene reacts very quickly with liquid bromine, in ...
Embodiment 2
[0041] (1) 20ml of Cr-Fe-Zn catalyst is loaded into a fixed-bed reactor, and the fixed-bed reactor is heated with an open-type tube heating furnace. Under the protection of 50ml of nitrogen, the catalyst was first dried at 10°C / min to 200°C for 2 hours, then raised to 400°C at a rate of 10°C / min and dried for 2 hours, then cooled to 330°C, and then passed through The catalyst was activated with 50 ml / min of hydrogen fluoride (HF) gas for 2 hours. This completes the catalyst activation process.
[0042]Heat the reactor to 350°C, then, 50ml / min tetrafluoroethane and 10ml / min nitrogen enter the mixing chamber together and mix well. After that, it goes through the reactor until buffer bottle, washing bottle, concentrated alkali solution, and liquid bromine absorption pool. After the experiment, the product is mainly distributed in the cooling collector. The collected product was subjected to GC analysis. GC results showed that the product contained 90.0% 1,2-dibromo-1,1,2-trif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com